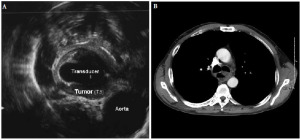
A 60 year-old man with a chief complaint of dysphagia was diagnosed with cancer of the esophagus in July 2009. Esophagogastroduodenoscopy (EGD) revealed an ulcerated lesion at 23-32 cm from the incisors, and the gastroesophageal junction was located at the 43 cm from the incisors. Biopsy confirmed invasive well to moderately differentiated squamous cell carcinoma of the mid-esophagus at the level of the carina. The malignancy, by endoscopic ultrasound, invaded beyond the muscularis propria layer into adjacent adventita (T3). It appeared to abut, though not clearly invade, the adjacent aorta (Fig. 1A). No tracheo-esophageal fistula was present at the time discernible by bronchoscopy. Computed tomography (CT) of the chest showed luminal narrowing of the esophagus at the level of the carina. There was some air and fluid in the more distal esophagus, which was mildly dilated (Fig. 1B). Further staging of the disease, by PET/CT, revealed the main lesion with SUVmax of 11, and a paraesophageal lymph node with SUVmax of 5.5. Malignancy was staged as cT3N1M0.
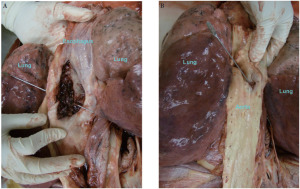
The patient received cisplatin/irinotecan (30/65 mg/m2) for the first dose of cycle 1, which was followed by complications of emesis, for which he received antiemetics and intravenous fluids for hydration. Consequently, the second dose of cycle 1 was delayed by one week. At this time, concurrent radiation treatment was started. At week 6, cycle 2 of cisplatin/irinotecan was started that led to recalcitrant emesis unrelieved by medications. Patient had persistent dysphagia and was nutritionally depleted. Subsequently, a percutaneous endoscopic gastrostomy (PEG) tube was inserted to supplement the patient’s nutritional requirements. Patient’s chemotherapy for the second dose of cycle 2 was postponed. At week 8, the patient was admitted for a presumed ileus and was unable to receive scheduled radiation treatments. At this point, he had received a total of 37.7 Gy in 21 fractions. On treatment day 60, patient arrived to the radiation medicine department to restart radiation treatments, but he was found to be tachycardic at 169 bpm and hypotensive at 50/33 mm Hg, with an O2 saturation of 80% on room air. He began to have evidence of bleeding at the skin margin of his PEG tube, as well as experiencing multiple episodes bright red hematemesis with clots totaling 400 cc. He was transferred to the Intensive Care Unit (ICU). Patient was intubated and an emergent endoscopy was performed that revealed bleeding from the site of malignancy. A through the scope (TTS) balloon was placed across the lesion, and inflated, in an attempt to tamponade bleeding. The patient went into ventricular tachycardia and failed resuscitative efforts. Autopsy was requested and revealed aorto-esophageal fistula to be the cause of death (Fig. 2A and B).
Primary aorto-esophageal fistula (AEF) is an uncommon event
(1, 2). Only 500 cases have been reported in the literature between 1928 and 1991. To put into perspective, this equates to roughly ten cases per year
(2). 54.2% of AEF are due to aneurysm rupture initiated by arteriosclerotic, syphilitic, or traumatic mechanisms
(3). Ingestion of foreign bodies (bones from animal foods, sharp metal objects) is the next common cause of aortic-esophageal fistulas at 19.2%. This is followed by esophageal malignancy (17.0%) and post-surgical fistula formation. Consequently, the yearly incidence is approximately one case associated with esophageal cancer. Chiari first describes the aortoesophageal fistula syndrome, as a painful radiation to the back, followed by a "signal hemorrhage", then a lucid interval (asymptomatic period)
(4, 5). Soon afterwards, overt exsanguinations can occur within hours to days later. One review states that 65% of AEF patients have sentinel bleed reported, and 59% of patients recall a history of chest pain
(2). However, very few AEF patients with an underlying esophageal malignancy present with all symptoms of the Chiari syndrome
(2). Our patient had sentinel hemorrhage without mid-thoracic pain, followed by immediate exsanguination after a short lucid interval of few minutes in the ICU.
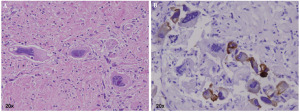
As for the formation of AEF, Postoloff
et al. along with other observers support that aortic perforation is caused by thrombosis of the vaso vasorum, accelerating the fistula formation between aorta and esophagus
(5-7). However, Postoloff reports three additional theories on esophageal perforation into the aorta
(8): i) invasion with most reported tumors seen only in the adventitia
(2); ii) bacterial infection
(9); iii) ulcerative process as tumor disintegrates
(10). On autopsy, our patient’s esophagus shows a deep ulceration with extensive necrosis and fibrosis involving the entire thickness of the esophageal wall, extending into the media of aorta. The ulcerative lesion of esophagus is measured to be 3.5 x 2.5 x 0.5 cm with a fistula tract between esophageal lesion and superior part of descending aorta, as seen grossly on the esophageal and aortic views in Figure 2A and 2B, respectively. Scattered atypical large cells, focally clustered, are seen within the area of necrosis, consistent with residual squamous cell carcinoma altered by chemo-radiation (Fig. 3A). On section immunoassays, these cells are positive for cytokeratin AE1/AE3 and are negative for both synaptophysin and neurofilament protein (Fig. 3B). However, no evidence of thrombosis in the vaso vasorum is observed, and other pathologic studies report similar findings
(2, 6, 8).
In this case, the formation of AEF is not through the thrombosis of vaso vasorum, but by the tumor’s ulcerative and infiltrative process. The early effect of chemo-radiation on normal tissues to accelerate fistula formation between the aorta and esophagus remains unclear, especially in the context of an infiltrating and ulcerative tumor in close proximity to the aorta. However, today, the majority of mid-thoracic esophageal cancers are treated with a multi-modality regimen, and the incidence of AEF still remains extremely rare. One would expect the incidence to be higher if chemo-radiation caused pathologic changes within normal tissues to form a fistula tract between the esophagus and the aorta. Whether or not multimodality regimen was initiated, our patient would have had the same poor outcome from the fistula formed by the tumor. If the diagnosis were made with enough time to treat, the decision whether to surgically repair the fistula should be individualized, according to the response of the tumor to the chemotherapy, patient’s general condition, and other operative risks. A Sengstaken esophageal balloon has been used either as a definitive treatment or as a temporizing measure before the definitive surgical procedure. Unfortunately, once the Chiari triad symptoms present, few patients have ever survived long enough to be treated.