Solitary gastric metastasis from primary lung adenocarcinoma: a rare site of extra-thoracic metastatic disease
Introduction
The commonest sites of extrathoracic spread of lung cancer are the supraclavicular and abdominal lymph nodes, liver, adrenal glands, brain, bone and skin (1). Metastases to gastrointestinal tract from lung cancer are uncommon with reported incidence ranging from 0.5% to 10% whereas the incidence of gastric metastasis ranges from 0.2% to 0.5% thus representing a rare event (2,3). GI metastases are mostly asymptomatic but when mucosal in location may cause symptoms and should be included in the differential diagnosis of any symptomatic lung cancer patient, as in this current case report. In this report, we present a patient who developed gastrointestinal bleeding caused by gastric metastasis of a primary lung cancer and briefly review the clinicopathological features and management of GI tract metastases due to advanced lung carcinomas.
Case report
A 61-year old male, heavy smoker (50 pack/years) was admitted to the authors’ department presenting persistent non-productive cough that had appeared two months previously. His past medical history was unremarkable. Upon admission, chest X-ray showed bilateral multiple nodular opacities with associated hilar lymphadenopathy (Figure 1). Further imaging evaluation with contrast-enhanced chest computed tomography revealed a mass measuring 2.7 cm with irregular edges in the apical segment of left upper lobe and bilateral multiple pulmonary nodules of different size as well as hilar lymphadenopathy. Fiberoptic bronchoscopy was performed demonstrating left apical segment stenosis by mucosal thickening. Transbronchial and deep submucosal biopsies documented adenocarcinoma since cancer cells were intensely positive for cytokeratin 7 (CK7) and thyroid-transcription factor-1 (TTF-1).
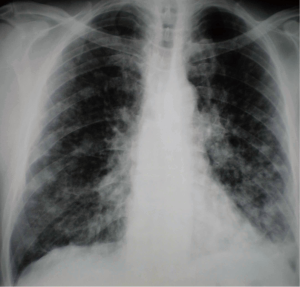
Additional work-up using abdominal CT detected secondary lesions in the left liver lobe. Brain CT was negative. Therefore, the clinical stage of the tumor was defined as stage IV(T4N3M1b).
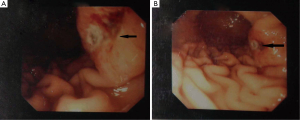
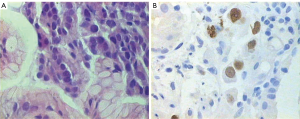
During hospitalization period, the patient developed bilateral swelling and tenderness at both lower extremities as well as two episodes of melena with associated considerable decrease in hematocrit value (Ht: 28%), albeit complete blood count was normal on admission day. Triplex ultrasonography of the lower extremities showed the presence of deep vein thrombus. Gastroduodenoscopy was then performed revealing an ulcerative lesion with coagulum on the posterior wall and greater curvature of the stomach (Figure 2). Biopsies from the lesion were obtained and pathological examination of specimens demonstrated adenocarcinoma (Figure 2A). To examine whether the gastric tumor was a primary cancer or a metastasis from the lung adenocarcinoma, immunohistochemistry was performed showing positive immunoreactivity for the markers TTF-1 and CK/7 and negative staining for CK/20 (Figure 3). Therefore, the diagnosis of gastric metastasis from lung adenocarcinoma was made.
Blood transfusion and intravenous omeprazole were given to the patient leading to complete response of the hematologic parameters and bleeding cessation. After hematocrit restoration, fondaparinux was administered subcutaneously for DVT treatment followed by combination chemotherapy including carboplatin, paclitaxel and bevacizumab. Shortly after three courses of chemotherapy (three months after diagnosis of single gastric metastasis), several metastatic lesions to brain and bones were detected by using contrast-enhanced brain CT and bone scitntigraphy. The patient’s general condition was deteriorated accordingly. Chemotherapy regimen was subsequently discontinued and palliative radiotherapy was applied. Because of his poor overall performance status, supportive care management was recommended without any supplementary therapeutic modality administration. He eventually succumbed to the disease approximately 10 months after his initial diagnosis.
Discussion
The stomach is an unusual site for metastasis. Breast, esophagus and malignant melanoma are the common primary metastatic sites, according to a recent large series of patients (2). Metastases to sites in the gastrointestinal tract from lung cancer are uncommon with reported incidence rate varying from 0.5% to 10%, as it has been demonstrated in autopsy series (3). The percentage, however, of gastric metastasis from lung carcinomas is estimated at 0.2-0.5% (4). Solitary lesions to the stomach in living patients were described sporadically as synchronous lesions at the time of lung cancer diagnosis or metachronous lesions after primary lung surgery (5-7) However, gastric metastasis is usually found in the presence of overwhelming metastatic burden. Lung cancer presenting with gastrointestinal involvement is generally considered to represent an advanced or end-stage disease (8).
Nevertheless, few cases of gastric and/or duodenal metastasis from various lung cancer cell types producing symptomatology have been described in the literature (5,6,7,9-13). The symptoms and signs arise from the growth of metastatic lesions involving mucosa whereas they do not occur in lesions located in the submucosal layer. The main clinical features include abdominal pain, anorexia, nausea, vomiting, anemia, hematemesis and melena. Furthermore, severe complications such as gastric perforation and pyloric obstruction have been reported in patients with gastric metastasis due to primary lung cancer. Intestinal involvement such as small and large bowel metastasis may present with hemorrhage and an acute abdomen as a result of perforation, obstruction and intussusception (14).
Lee et al. have recently shown that the median duration from lung cancer diagnosis to GI metastasis was three months and the average time from diagnosis of GI metastasis to death was 2.8 months, similar data to those mentioned in previous studies (15,16). Moreover, no significant difference was observed in overall survival in patients with initial stage I-III lung cancer upon GI metastasis diagnosis in comparison with those with stage IV thus demonstrating that GI metastases from lung cancer may portend poor prognosis. Every histological type of lung cancer can cause GI metastasis but adenocarcinoma and squamous cell carcinoma can metastasize more frequently to the gastrointestinal tract than any other lung cancer cell type.
In general, the use of abdominal sonography and CT might have a role in identifying gastric metastasis. However, positron emission tomography PET/CT scan is the most common investigative and effective tool in detecting GI metastases, both symptomatic or not (17,18). The combined use of endoscopic ultrasonography (EUS) and PET-CT seems to be an ideal modality in the preoperative staging of gastric cancer, according to the results of a recent study (7,19). Nevertheless, endoscopy is an essential tool for identifying lesions when the patients present with symptoms relevant to the gastrointestinal tract. Several patterns have been described such as solitary or multiple polypoid submucosal masses, which may ulcerate and infiltrating constricting pattern similar to a “linitis plastica” (9,15).
However, endoscopic findings are non-specific to differentiate metastatic gastric cancer due to lung tumors from primary gastrointestinal cancer. Hence, immunohistochemistry provides a valuable and reliable method in distinguishing primary lung tumors from metastatic tumors to the lung from common sites (colon, breast, prostate, pancreas, stomach, kidney, bladder, ovaries, and uterus) (20). In particular, several different keratins have been employed to subclassify primary lung tumors but the most popular of them are CK7 and CK20. It has already been demonstrated that primary lung carcinomas usually express the immunophenotype of CK7+/CK20-, whereas gastrointestinal carcino¬mas have the CK7-/CK20+ pattern (8). Strictly speaking, CK7+/CK20- immunophenotype is seen in 90-100% of patients with primary lung cancer. However, this pattern has been observed in 45% of patients with gastrointestinal cancers such as primary rectal or small bowel adenocarcinomas (16). Thus, to rule out this eventuality, using TTF-1 in combination with markers CK7 and CK20 could lead to the differentiation of metastatic GI tumors from lung cancer with reasonable degree of certainty. TTF-1 is highly specific for adenocarcinomas of pulmonary origin exhibiting a positive predictive value of 100% (8,20). In the present case, both lung and gastric cancerous lesions were positive for CK7 and TTF-1 and negative for CK20 suggesting lung as the primary site of adenocarcinoma.
Therapeutic approach should initially include conservative measures (e.g., fluid resuscitation, blood transfusion, medication reducing gastric acidity) and endoscopy-based interventions for bleeding control (e.g., electrocoagulation, laser, epinephrine injection). The role of surgery in the management of gastrointestinal metastases due to primary lung cancer does not appear controversial given the high reported 100% perioperative mortality for gastric and duodenal symptomatic metastatic disease and the poor outcomes (15). Rather, surgery should be reserved for solitary metastatic disease to the stomach, or for cases with severe bleeding, obstruction or perforation when conservative or endoscopic interventions are not possible (21). In the present case, upon diagnosis of stomach involvement, conservative treatment was prescribed. Surgical procedure was not performed since the symtoms were effectively controlled by medication and the patient’s general condition was poor with other simultaneous metastases thus rendering surgical management problematic and unavailing.
In conclusion, gastrointestinal tract metastases from lung cancer signify a late-stage disease and should be considered in the differential diagnosis of lung cancer patients presenting with an acute abdomen and gastrointestinal bleeding. Immunohistochemistry is very useful tool in differentiating between primary lung cancer metastasizing to gastrointestinal tract and metastatic GI tumors in equivocal cases. Surgical therapy is not usually indicated for metastatic GI lesions originated from lung cancer due to their unfavorable outcome. However, surgical intervention is typically necessitated to prevent life-threatening GI events such as bleeding, obstruction and perforation thus providing effective palliation as well as long-term survival in patients with only a solitary GI metastasis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Navani N, Spiro SG. Symptoms and signs of lung cancer. In: Spiro SG, Huber RM, Janes SM. (eds). Thoracic Malignancies. Eur Respir Mon 2009;44:71-87.
- De Palma GD, Masone S, Rega M, et al. Metastatic tumors to the stomach: Clinical and endoscopic features. World J Gastroenterol 2006;12:7326-28.
- Antler AS, Ough Y, Pitchumoni CS, et al. Gastrointestinal metastases from malignant tumors of the lung. Cancer 1982;49:170-2.
- Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer 1990;65:1596-600.
- Ozdilekcan C, Songür N, Memiş L, et al. Lung cancer associated with a single simultaneous solitary metastatic lesion in stomach: a case report with the review of literature. Tuberk Toraks 2010;58:78-84.
- Aokage K, Yoshida J, Ishii G, et al. Long-term survival in two cases of resected gastric metastasis of pulmonary pleomorphic carcinoma. J Thorac Oncol 2008;3:796-9.
- Sileri P, D'Ugo S, Blanco Gdel V, et al. Solitary metachronous gastric metastasis from pulmonary adenocarcinoma. Report of a case. Int J Surg Case Rep 2012;3:385-8.
- Rossi G, Marchioni A, Romagnani E, et al. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol 2007;2:115-20.
- Okazaki R, Ohtani H, Takeda K, et al. Gastric metastasis by primary lung adenocarcinoma. World J Gastrointest Oncol 2010;2:395-8.
- Casella G, Di Bella C, Cambareri AR, et al. Gastric metastases by lung small cell carcinoma. World J Gastroenterol 2006;12:4096-7.
- Suzaki N, Hiraki A, Ueoka H, et al. Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Res 2002;22:1209-12.
- Altintas E, Sezgin O, Uyar B, et al. Acute upper gastrointestinal bleeding due to metastatic lung cancer: an unusual case. Yonsei Med J 2006;47:276-7.
- Hamatake M, Ishida T, Yamazaki K, et al. Lung cancer with p53 expression and a solitary metastasis to the stomach: a case report. Ann Thorac Cardiovasc Surg 2001;7:162-5.
- Garwood RA, Sawyer MD, Ledesma EJ, et al. A case and review of bowel perforation secondary to metastatic lung cancer. Am Surg 2005;71:110-6.
- Lee PC, Lo C, Li MT, et al. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol 2011;17:4314-20.
- Yang CJ, Hwang JJ, Kang WY, et al. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer 2006;54:319-23.
- Shomura H, Nakano S, Funai T, et al. A case of metastasis to the stomach from primary adenocarcinoma of the lung cancer. Gan To Kagaku Ryoho 2010;37:2481-3.
- Cronin CG, Scott J, Kambadakone A, et al. Utility of positron emission tomography/CT in the evaluation of small bowel pathology. Br J Radiol 2012;85:1211-21.
- Li B, Zheng P, Zhu Q, et al. Accurate preoperative staging of gastric cancer with combined endoscopic ultrasonography and PET-CT. Tohoku J Exp Med 2012;228:9-16.
- Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med 2008;132:384-96.
- Goh BK, Yeo AW, Koong HN, et al. Laparotomy for acute complications of gastrointestinal metastases from lung cancer: is it a worthwhile or futile effort? Surg Today 2007;37:370-4.


