Acute myeloid leukemia masquerading as hepatocellular carcinoma
Case presentation
A 64-year-old Caucasian man developed progressive fatigue, abdominal distension, and 25 pounds weight loss (10% of his body weight) over a month period. His outpatient workup included an abdominal ultrasound that revealed a large liver mass. Magnetic resonance imaging confirmed a solitary 11 cm mass with central necrosis within segments 6 and 7 of the right hepatic lobe. The patient had no history of excessive alcohol intake, viral hepatitis, steatosis or liver cirrhosis. An alpha-fetoprotein (AFP) level was 7.9 ng/mL. Given the features of the liver mass on imaging, and despite the lack of cirrhosis and elevated AFP, a diagnosis of hepatocellular carcinoma (HCC) was presumed and the patient was considered for hepatobiliary surgery.
In the interim, the patient had rapid functional decline. He was admitted for fever (39 °C), confusion and back pain. The physical exam was notable for altered mental status and flapping tremor without meningismus, lower extremity weakness or focal neurologic findings. Laboratory studies revealed a white blood count (WBC) of 3.0×103/µL, hemoglobin of 10.3 g/dL and platelets of 78,000/µL. Peripheral blood smear showed a few myelocytes and metamyelocytes with blunt pseudopods. Other notable findings included corrected serum calcium of 12.4 mg/dL, AST of 56 U/L, ALT of 42 U/L, serum ammonia of 65 mcg/dL, and an LDH of 3,304 U/L. A lumbar spinal MRI revealed extensive marrow space abnormality suspicious for metastatic disease. A subsequent bone marrow biopsy, from the right posterior iliac crest, was necrotic and non-diagnostic.
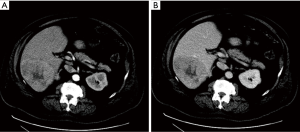
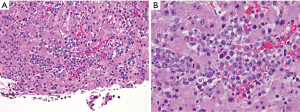
A computed tomography (CT) scan of the abdomen without and with late arterial-phase and portal venous-phase contrast demonstrated arterial phase hypervascularity of the previously described 11 cm mass with central necrosis and portal phase washout on a background non-cirrhotic liver (Figure 1). A CT-guided core biopsy of the liver mass revealed sinusoidal infiltration and focal architectural effacement by a population of immature mononuclear cells suggestive of a monocytic/monoblastic myeloid sarcoma (MS) (Figure 2).
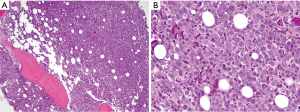
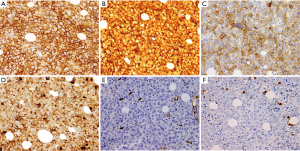
Flow cytometry revealed an abnormal immature hematopoietic population representing only 0.1% of WBCs and expressing CD13, CD33, CD117 with increased forward and side scatter (larger, granular cells of myelomonocytic lineage). A repeat bone marrow biopsy from the left posterior iliac crest showed areas of hypercellular marrow (>90%) with 52% blasts of monocytic lineage (Figure 3). Imunnohistochemical analysis revealed a characteristic staining pattern positive for CD13, CD33, CD68, CD117, CD163, CD56 and lysozyme (Figure 4A-D), but negative for myeloperoxidase (MPO) and CD34 (Figure 4E,F). A diagnosis of acute monocytic/monoblastic leukemia was established. Gene mutation analysis revealed a Janus Kinase family (JAK) 1 (V568I), TET2 (P1012L), and TP53 (Y236C) mutations. Fluorescence in situ hybridization (FISH) revealed absence of mixed lineage leukemia (MLL) gene rearrangement. A karyotype was not obtainable.
Shortly after, induction chemotherapy with cytarabine and idarubicin was initiated. Patient’s AML was refractory to several lines of chemotherapy and he succumbed to complications of his extensive disease 4 months later.
Discussion
The differential diagnosis of a liver mass is broad. In the appropriate clinical context as part of the validated screening programs, and in the presence of cirrhosis and an elevated AFP, the diagnosis of HCC can be made based on non-invasive radiologic criteria. This involves imaging techniques obtained by 4-phase multidetector CT scan or dynamic contrast-enhanced MRI. The findings of heterogeneous arterial phase hyper-enhancement followed by washout appearance is highly suggestive of HCC in the cirrhotic liver and at this size may be diagnostic of HCC in the absence of an extrahepatic primary tumor known to produce hypervascular metastases (1-3). Tumor biopsy is often unnecessary with such lesions of larger size prior to surgical resection (4).
It is important to emphasize, however, that HCC may arise in the absence of cirrhosis, typically in older patients who are chronic hepatitis B carriers (5). In this context, imaging studies cannot be used to establish the diagnosis. As exemplified by this case, a pathology diagnosis must be obtained in the absence of cirrhosis or other HCC risk factors. The extended differential diagnosis based on radiographic appearance in this case includes hypervascular metastases such as neuroendocrine tumor, hepatocellular adenoma, cholangiocarcinoma, and fibrolamellar carcinoma. Lymphomatous hepatic masses generally enhance minimally as they are not typically hypervascular, while leukemic hepatic masses have not been well described in large imaging series.
A tumor consisting of myeloid blasts at an extramedullary site is known as a MS or chloroma (6-8). Case series of MS provide insight into the multifaceted clinical manifestations and varied histopathologic and cytogenetic characteristics of extramedullary AML (9,10). It is often of myelomonocytic or monoblastic morphology (7,9-10), consistent with the infiltrative and extravascular function of the monocyte lineage. Immunohistochemistry (IHC) is most commonly positive for CD68 followed by MPO and CD117 (9). Cytogenetic abnormalities associated with extramedullary AML include MLL rearrangement, t(8:21), inversion 16, 11q abnormalities, and mutations of nucleophosmin (NPM) 1 (9,10). Mutations in the JAK, TET2, and TP53 found in this case have been described in the pathogenesis of AML, but not in relation to MS (9-14).
MS may present de novo, preceding AML, or concurrently with AML (7). However, it remains uncommon (less than 1%) for prominent extramedullary disease to present first (8). With concurrent bone marrow involvement, the more common extramedullary sites of leukemic infiltration include the skin and gingiva. On the other hand, isolated MSs commonly involve the bone, soft tissue, lymph nodes, gastrointestinal tract, mediastinum and gonads (7,10,15). Several other anatomic sites of MSs have been reported, but the liver has only been involved in a minority of cases (10,16-18).
Previous case reports have described MS mimicking hepatobiliary malignancies in their presentation. While some cases had an extra-hepatic biliary ductal lesion appearing similar Klatskin tumors (16-18), others were associated with diffuse myeloid infiltration of liver sinusoids without a definitive liver mass (19-20). To our knowledge, there are no reported cases of de novo AML presenting as a MS that mimics focal HCC in its radiographic appearance.
Given the confirmed diagnosis of acute monocytic/monoblastic leukemia, the patient was started on standard induction therapy for AML with cytarabine and idarubicin. MS occurring de novo should be considered as AML and treated as such (7). A limited number of studies evaluate the prognostic impact of MS. While some report a negative prognosis in certain subgroups (21,22), others suggest a non-inferior outcome after conventional chemotherapy or allogeneic stem cell transplant in comparison to ‘typical’ AML (23,24). The decision to proceed with allogeneic stem cell transplant as opposed to conventional chemotherapy is mostly hinged upon the clinical features suggestive of aggressive disease. Surgical debulking or radiation therapy of MS may be considered when the lesion is causing compression of vital organs, but there is no evidence of superiority of this combined approach compared to chemotherapy alone (25).
The presentation of extramedullary AML is indeed multifaceted and can be mistaken for a variety of other malignancies including hepatobiliary tumors and HCC. This case demonstrates that histologic confirmation is required to make the diagnosis of HCC in a patient with no HCC risk factors, and particularly in a non-cirrhotic liver.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Parente DB, Perez RM, Eiras-Araujo A, et al. MR imaging of hypervascular lesions in the cirrhotic liver: a diagnostic dilemma. Radiographics 2012;32:767-87. [Crossref] [PubMed]
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Levy I, Greig PD, Gallinger S, et al. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg 2001;234:206-9. [Crossref] [PubMed]
- Do AL, Wong CR, Nguyen LH, et al. Hepatocellular carcinoma incidence in noncirrhotic patients with chronic hepatitis B and patients with cirrhosis of all etiologies. J Clin Gastroenterol 2014;48:644-9. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2008.
- Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453-74. [Crossref] [PubMed]
- Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood 2012;119:34-43. [Crossref] [PubMed]
- Campidelli C, Agostinelli C, Stitson R, et al. Myeloid sarcoma: extramedullary manifestation of myeloid disorders. Am J Clin Pathol 2009;132:426-37. [Crossref] [PubMed]
- Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma 2006;47:2527-41. [Crossref] [PubMed]
- Xiang Z, Zhao Y, Mitaksov V, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood 2008;111:4809-12. [Crossref] [PubMed]
- Tomasson MH, Xiang Z, Walgren R, et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood 2008;111:4797-808. [Crossref] [PubMed]
- Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 2012;119:2114-21. [Crossref] [PubMed]
- Gaidzik VI, Paschka P, Späth D, et al. TET2 mutations in acute myeloid leukemia (AML): results from a comprehensive genetic and clinical analysis of the AML study group. J Clin Oncol 2012;30:1350-7. [Crossref] [PubMed]
- Choi EK, Ha HK, Park SH, et al. Granulocytic sarcoma of bowel: CT findings. Radiology 2007;243:752-9. [Crossref] [PubMed]
- Lee JY, Lee WS, Jung MK, et al. Acute myeloid leukemia presenting as obstructive jaundice caused by granulocytic sarcoma. Gut Liver 2007;1:182-5. [Crossref] [PubMed]
- Rajesh G, Sadasivan S, Hiran KR, et al. Acute myeloid leukemia presenting as obstructive jaundice. Indian J Gastroenterol 2006;25:93-4. [PubMed]
- Mano Y, Yokoyama K, Chen CK, et al. Acute myeloid leukemia presenting with obstructive jaundice and granulocytic sarcoma of the common bile duct. Rinsho Ketsueki 2004;45:1039-43. [PubMed]
- Wandroo FA, Murray J, Mutimer D, et al. Acute myeloid leukaemia presenting as cholestatic hepatitis. J Clin Pathol 2004;57:544-5. [Crossref] [PubMed]
- Hang XF, Xin HG, Wang L, et al. Nonleukemic myeloid sarcoma of the liver: a case report and review of literature. Hepatol Int 2011;5:747-50. [Crossref] [PubMed]
- Byrd JC, Weiss RB, Arthur DC, et al. Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): results from Cancer and Leukemia Group B 8461. J Clin Oncol 1997;15:466-75. [PubMed]
- Reinhardt D, Pekrun A, Lakomek M, et al. Primary myelosarcomas are associated with a high rate of relapse: report on 34 children from the acute myeloid leukaemia-Berlin-Frankfurt-Münster studies. Br J Haematol 2000;110:863-6. [Crossref] [PubMed]
- Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer 2008;113:1370-8. [Crossref] [PubMed]
- Chevallier P, Mohty M, Lioure B, et al. Allogeneic hematopoietic stem-cell transplantation for myeloid sarcoma: a retrospective study from the SFGM-TC. J Clin Oncol 2008;26:4940-3. [Crossref] [PubMed]
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood 2011;118:3785-93. [Crossref] [PubMed]


