The role of local excision in invasive adenocarcinoma of the ampulla of Vater
Background
Tumors of the ampulla of Vater represent a small portion of periampullary tumors, accounting for less than 1% of all gastrointestinal malignancies (1,2). Though these tumors are recognized as adenocarcinomas, they have been reported to carry a more favorable prognosis than pancreatic or distal biliary malignancies, with 5-year survival rates ranging from 20% to 50% (3,4). For patients without metastatic disease, standard therapy entails radical surgical resection (pancreaticoduodenectomy), though a few contemporary reports have discussed the viability of local resection (ampullectomy) for select groups of patients. Outcomes in one small cohort of patients with small, favorable histology tumors treated by local resection demonstrated a 5-year overall survival of 33% (5). Other studies which included patients with more advanced tumors treated with local resection, however, are handicapped by small patient numbers (5-9). Consequently, the role of local resection for invasive ampullary adenocarcinomas remains ill-defined. This study reports the outcomes of a relatively large, single-institutional series of patients who underwent open ampullectomy for invasive adenocarcinoma of the ampulla of Vater in patients either deemed unsuitable for or refusing pancreaticoduodenectomy.
Methods
Medical records of all patients treated at Duke University Hospitals who were diagnosed with adenocarcinoma of the ampulla of Vater from 1976 to 2010 were analyzed with Institutional Review Board (IRB) approval. All patients in this series were thought to potentially benefit from pancreaticoduodenectomy, but based on either medical comorbidities (with associated high risk of operative morbidity and mortality) or patient preference, local excision was carried out. At the time of evaluation, all procedures were conducted with curative intent. Only adenocarcinomas originating from the ampulla of Vater were included, excluding those found to have bile duct, pancreas, and duodenal primaries. Patients who underwent radical resection or were found to have metastatic disease were also excluded from the study.
Surgery
All patients underwent open ampullectomy using a technique as described in detail by Clary et al. (10). Briefly, following exploration through a subcostal or midline incision, a Kocher maneuver was performed. The ampullary mass was palpated through the duodenal wall and a lateral duodenotomy performed opposite the site of the mass. The ampulla was identified following stay suture placement on the duodenal wall, and subsequently circumferential resection of the duodenal mucosa and mass performed. All surgical specimens were staged based on the American Joint Committee on Cancer 7th edition staging manual. Pathology data pertaining to tumor grade, T staging, and surgical margin status were collected and reviewed at Duke University Hospital. For patients whose original pathology report did not note the grade or T stage, specimens were reassessed for the purposes of this study. Two patients were determined to have clinical T3 disease based on pancreatic involvement seen on CT scan and endoscopic ultrasound, respectively.
Chemoradiotherapy
The decision to deliver adjuvant therapy was based on surgical margins, T and N stage, grade, and presence of lymphovascular invasion. All patients found to have involved surgical margins were offered adjuvant therapy. Multifield external beam radiation therapy was used to target the tumor bed and local-regional lymph node basins, which include celiac, porta hepatis, superior mesenteric artery, and pancreaticoduodenal nodes. Patients received 5 treatments per week at 1.8-2 Gy per fraction to a total dose of 45.0-50.4 Gy. Field arrangements were primarily antero-posterior/postero-anterior in addition to opposed lateral fields. Patients underwent 3-dimensional treatment planning starting in 1997. The use of adjuvant chemotherapy was determined by the patients’ medical oncologist. All patients who received chemotherapy received fluoropyrimidine-based treatment concurrent with radiation therapy.
Statistical analysis
The goal of this study was to assess local control (LC), disease-free survival (DFS), metastasis-free survival (MFS) and overall survival (OS) rates following local resection. Failure patterns were analyzed during clinical follow-ups, using radiological imaging, biopsy, and endoscopy. Disease recurrence in the tumor bed or local-regional lymph nodes (celiac, pancreaticoduodenal, superior mesenteric artery basins, and porta hepatis) were defined as local failures. Recurrences outside these areas were defined as distant failures. LC and MFS were calculated from date of surgery to local or distant failures, respectively. Disease-free survival was calculated from date of surgery to first failure or death, whichever came first. Overall survival was calculated from date of surgery to death due to any cause. Times to event endpoints were estimated using the Kaplan-Meier method and analyzed by the statistics department at the Duke Cancer Institute. Patient follow-ups were generally every 3 months following their treatment completion.
Results
Seventeen patients underwent open ampullectomy for localized, invasive ampullary carcinoma at Duke University between 1976 and 2010. Patients’ median age was 72 years, with a mean follow-up time for all patients of 2.94 years. Presenting symptoms frequently included jaundice, abdominal pain, and pancreatitis.
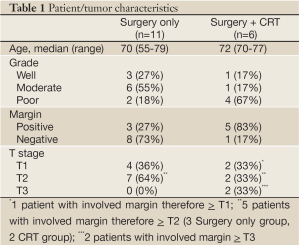
Patient characteristics are summarized in Table 1. There were no perioperative deaths. Rare postoperative complications included wound infections, cholangitis, and adhesive gastrointestinal obstruction. Eleven patients (65%) had T2 disease or higher while 6 patients (35%) had T1 disease. Thirteen patients (76%) had moderate or poor histological grade, while 8 patients (47%) had involved surgical margins. All but one patient receiving adjuvant chemoradiation therapy (CRT) had involved surgical margins, while 5 of 8 (63%) patients with involved margins received adjuvant therapy. Similarly, a higher proportion of patients who received CRT had poorly differentiated histology (67% vs. 18%) and more advanced tumors, although no statistical significance was found due to small sample size. These patients received a median radiation dose of 45 Gy, with infusional 5-fluorouracil or capecitabine delivered concurrently in all but one patient. All patients who received radiation therapy completed the full prescribed course. No patient received adjuvant chemotherapy alone or additional chemotherapy following adjuvant chemoradiotherapy.
Full table
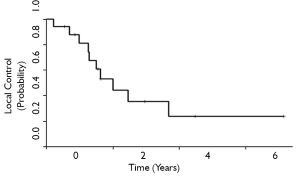
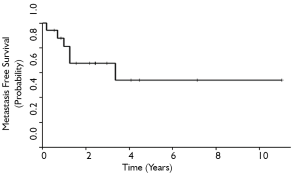
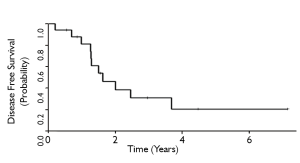
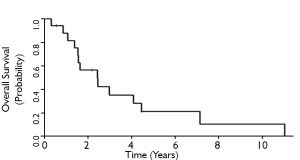
The 3-and 5-year local control rates were 36% and 24%, respectively for all patients who underwent ampullectomy (Figure 1). Local recurrence appeared to be lower for T1 tumors, although statistical comparisons are limited by sample size. 10 patients (63%) experienced local failure, 7 of which received surgery alone and 3 who received adjuvant therapy. Locally recurrent patients frequently presented with symptoms of obstructive jaundice, nausea, anorexia, right upper quadrant tenderness, and generalized fatigue. The 3-and 5-year MFS rates for all patients were 68% and 54%, respectively (Figure 2). Six patients (35%) experienced distant failures (2 patients who received surgery alone and 4 who underwent adjuvant therapy). Three-and 5-year DFS rates were 31% and 21%, respectively, while the 3-and 5-year OS rates were 35% and 21%, respectively (Figures 3,4). The group which received adjuvant CRT did not appear to have better LC, MFS, or DFS compared with surgery alone, although this group tended to have poorer histological grade, more advanced tumor staging and involved surgical margins.
Discussion
Adenocarcinoma of the ampulla of Vater is an uncommon malignancy that accounts for less than 1% of all gastrointestinal malignancies (1,2). Most of these tumors contain adenomatous tissue, suggesting that the majority arise from ampullary adenomas (11). As opposed to pancreatic adenocarcinoma, most patients with ampulla of Vater cancer present as with potentially resectable disease, possibly due to earlier presentation of clinical symptoms and a more indolent tumor biology (12). Some authors have recommended the standard treatment approach for ampullary tumors, including benign disease, consist of radical resection (pancreaticoduodenectomy), given potential difficulty in excluding malignancy with preoperative biopsy and a high tendency for recurrence following local excision alone (13,14). However, given pancreaticoduodenectomy carries significant morbidity and mortality, other authors have reported local resection (ampullectomy) to be a treatment option in select patients with benign or small tumors (15-17). While radical surgery has been the standard treatment for invasive ampullary adenocarcinomas at our institution, the present study sought to evaluate outcomes and probability of failure following local resection for select patients who were not candidates for or who refused pancreaticoduodenectomy.
Previous studies investigating outcomes of patients undergoing pancreaticoduodenectomy alone for ampullary cancers have reported 5-year local control and overall survival rates ranging from 50-83% and 36-67%, respectively. When surgery is combined with adjuvant CRT, reported 5-year local control and overall survival rates of 73-80% and 35-60%, respectively, have been observed (18-22). These studies have indicated disease-related outcomes correlate strongly with tumor and nodal staging, tumor differentiation, and margin status. These reports have also highlighted the importance of achieving local control in this disease and its impact on overall survival. Given that ampullectomy results in a reduction in surgical margin as well as a lack of clearance of locoregional lymph nodes as compared with radical resection, it is reasonable to assume that additional failures would manifest as local recurrence.
Our study showed a 5-year LC rate of 24% and an OS of 21% for patients undergoing ampullectomy, with or without CRT. These values are worse than those reported in patients who have undergone radical resection, though statistical comparison is not feasible. The high rates of local failure associated with local resection may be explained by the presence of subclinical locoregional disease outside the area of resection. While these factors are inherent in any type of surgical resection, including pancreaticoduodenectomy, our study suggests that this occurs much more frequently with ampullectomy compared to radical resection. Accordingly, 53% of our patients were found to have marginal involvement on pathology. Given that the majority of the patients in our study were found to have positive surgical margins, our outcomes may suggest an underestimation of the true residual disease burden for these patients. We identified some variability in the anatomy of our ampullectomy specimens; many of the specimens in our study terminated in the duodenal wall, allowing for a maximal pathologic stage pT2 assignment. In these cases, the presence of positive surgical margins suggests the true pathologic T stage could be higher. It is possible extension of the ampullectomy into the pancreatic parenchyma could potentially allow for more complete (R0) resection of T2 and even some T3 tumors. In addition, the lack of regional lymph nodes in ampullectomy specimens precludes pathologic N-staging. If we assume that some our patients had pT3-T4 and/or pN1 disease at the time of their resection, higher rates of local recurrence would be anticipated. Additionally, the high rates of local failure and poor outcomes in our series suggest that ampullectomy does not offer satisfactory local regional disease control and may not serve as a viable option for curative resection for patients with invasive disease in all but very highly selected patients.
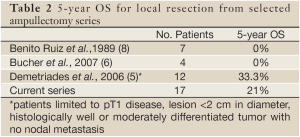
Few studies have evaluated patients undergoing local resection for ampullary adenocarcinomas, reporting 5-year overall survival rates ranging from 0 to 33% (Table 2) (5,6,8). None of these has employed the use of adjuvant CRT. Our study demonstrated results consistent with prior studies, offering a relatively larger patient population. None of the patients survived beyond 5 years in the studies reported by Ruiz et al. and Bucher et al. (6,8). Our 5-year OS rates are lower than those reported by Demetriades et al., which may be explained by their inclusion of only patients with well or moderately differentiated pT1 tumors less than 2 cm in diameter (5). Given it has been reported that lymph node involvement increases from 9% in pT1 to 50% in pT2 tumors (23), it is therefore not surprising that our series, which included 65% of patients with T2 disease or higher, yielded a lower 5-year OS rate of 21%. Since our cohort size was limited, our statistical analysis did not directly compare survival by T stage. However, subset analysis demonstrated a 40% 5-year survival for patients with T1 disease, compared with 16% and 0% for T2 and T3 disease, respectively. Thus, the relatively favorable prognosis for patients with T1 disease may suggest the procedure to be an option for highly selected patients.
Full table
This study has several limitations associated with retrospective studies. Patients who received CRT displayed higher rates of positive margins and poor tumor differentiation, demonstrating selection bias. Consistent with other similar types of retrospective studies, patients with more advanced disease are generally referred for CRT. Furthermore, the rarity of ampullary adenocarcinomas, in conjunction with the subset of patients who are unfit or refuse radical resection, yielded only 17 patients over 34 years at our institution. Though our sample size is limited, to our knowledge, it represents the largest reported series of patients with invasive ampullary adenocarcinoma managed by local resection.
Given there are few series evaluating the treatment of ampullary malignancies by local resection, there is a lack of coherent criteria for defining when local excision is suitable for invasive adenocarcinomas. Given the rarity of ampullary carcinomas and a lack of randomized prospective studies, large institutional experiences can facilitate treatment planning. These findings suggest that given the high rates of local failure and poor overall survival, local resection with ampullectomy is not an adequate method of curative resection in the vast majority of invasive tumors, even in combination with adjuvant chemoradiation therapy.
In summary, our series suggests that ampullectomy for invasive ampullary adenocarcinomas is a relatively safe procedure but does not offer satisfactory long-term results, mostly due to high local failure rates. Adjuvant chemoradiation therapy does not appear to offer increased local control or survival benefit following ampullectomy, although these results may suffer from selection bias and small sample size. We believe that local resection should be limited to benign ampullary lesions or patients with very small, early tumors with favorable histologic features where pancreaticoduodenectomy is not deemed feasible. Additionally, ampullectomy can serve as a diagnostic procedure to provide frozen section analysis to evaluate for the presence of invasive carcinoma, following which pancreaticoduodenectomy can be performed (10). Although our study suggests low cure rates for patients with more advanced ampullary tumors, local resection, possibly combined with chemoradiotherapy, may serve as safe and adequate approach to palliation as well as a chance of long-term disease-free survival for a small number of patients who are not operative candidates or who refuse pancreaticoduodenectomy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Branum GD, Pappas TN, Meyers WC. The management of tumors of the ampulla of Vater by local resection. Ann Surg 1996;224:621-7.
- Howe JR, Klimstra DS, Moccia RD, et al. Factors predictive of survival in ampullary carcinoma. Ann Surg 1998;228:87-94.
- Michelassi F, Erroi F, Dawson PJ, et al. Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Ann Surg 1989;210:544-54; discussion 554-6.
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447-58.
- Demetriades H, Zacharakis E, Kirou I, et al. Local excision as a treatment for tumors of ampulla of Vater. World J Surg Oncol 2006;4:14.
- Bucher P, Chassot G, Durmishi Y, et al. Long-term results of surgical treatment of Vater’s ampulla neoplasms. Hepatogastroenterology 2007;54:1239-42.
- Nobili P, Mancini S, Annolfi B, et al. Surgical treatment of tumors of the ampulla of Vater by local resection. G Chir 1998;19:478-84.
- Benito Ruiz J, Del Pino Porres J, Herrero Bernabéu C. Tumors of Vater’s ampulla. Review of 25 cases. Rev Esp Enferm Apar Dig 1989;76:144-50.
- Chiappetta A, Sperti C, Bonadimani B, et al. Surgical experience with adenocarcinoma of the ampulla of Vater. Am Surg 1986;52:603-6.
- Clary BM, Tyler DS, Dematos P, et al. Local ampullary resection with careful intraoperative frozen section evaluation for presumed benign ampullary neoplasms. Surgery 2000;127:628-33.
- Kozuka S, Tsubone M, Yamaguchi A, et al. Adenomatous residue in cancerous papilla of Vater. Gut 1981;22:1031-4.
- Sikora SS, Balachandran P, Dimri K, et al. Adjuvant chemo-radiotherapy in ampullary cancers. Eur J Surg Oncol 2005;31:158-63.
- Chareton B, Coiffic J, Landen S, et al. Diagnosis and therapy for ampullary tumors: 63 cases. World J Surg 1996;20:707-12.
- Beger HG, Treitschke F, Gansauge F, et al. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg 1999;134:526-32.
- Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248-57; discussion 257-60.
- Böttger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg 1999;23:164-71; discussion 171-2.
- Tarazi RY, Hermann RE, Vogt DP, et al. Results of surgical treatment of periampullary tumors: a thirty-five-year experience. Surgery 1986;100:716-23.
- Narang AK, Miller RC, Hsu CC, et al. Evaluation of adjuvant chemoradiation therapy for ampullary adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Radiat Oncol 2011;6:126.
- Krishnan S, Rana V, Evans DB, et al. Role of adjuvant chemoradiation therapy in adenocarcinomas of the ampulla of vater. Int J Radiat Oncol Biol Phys 2008;70:735-43.
- Kim K, Chie EK, Jang JY, et al. Role of adjuvant chemoradiotherapy for ampulla of Vater cancer. Int J Radiat Oncol Biol Phys 2009;75:436-41.
- Zhou J, Hsu CC, Winter JM, et al. Adjuvant chemoradiation versus surgery alone for adenocarcinoma of the ampulla of Vater. Radiother Oncol 2009;92:244-8.
- Palta M, Patel P, Broadwater G, et al. Carcinoma of the Ampulla of Vater: Patterns of Failure Following Resection and Benefit of Chemoradiotherapy. Ann Surg Oncol 2012;19:1535-40.
- Yoon YS, Kim SW, Park SJ, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg 2005;242:92-100.