
Stereotactic body radiation therapy for the treatment of locally recurrent pancreatic cancer after surgical resection
Introduction
Pancreatic cancer is the 8th most common cancer and the 3rd most common cause of cancer deaths in the United States, accounting for approximately 48,000 deaths each year (1). The incidence of pancreatic cancer is rising and is expected to be the 2nd most common cause of cancer deaths by the year 2030 (2). Management of pancreatic cancer is dependent on disease extent and usually involves a combination of surgery, chemotherapy, and radiation therapy (3). After aggressive multimodality therapy, outcomes are guarded, with 5-year overall survival (OS) rates of less than 15% for localized disease (4).
Although most patients fail distally after resection of localized pancreatic cancer, a significant proportion can develop locoregional relapse (5,6). In fact, the first site of failure after curative resection can be locoregional in up to 45–50% of recurrences (7,8). Furthermore, uncontrolled local progression can lead to a various complications, which in turn, can negatively impact morbidity and mortality outcomes (9,10). As systemic therapy continues to improve, allowing patients to live longer, it is expected that the incidence of locoregional failures may rise, highlighting the need for further investigation into the management of locoregional relapse (11,12).
The optimal treatment of locoregional failure following initial curative resection is unknown. Management options include re-resection, radiation therapy, chemotherapy, or combination therapy. Several studies have shown that re-resection of local recurrences can significantly improve outcomes when compared to unresected disease (13-15). Unfortunately, most patients are not suitable for re-resection because of medical co-morbidities and/or technically unresectable disease. Non-invasive local therapies such as stereotactic body radiation therapy (SBRT) may be an appealing option in these situations. Although a few reports have investigated SBRT for locally recurrent pancreatic cancer, limitations of these studies include small sample size and heterogeneous patient populations, such as including previously irradiated or unresected disease (16-20). As such, we report on clinical outcomes and toxicity in a cohort of radiation-naïve patients who was treated with SBRT for locally recurrent pancreatic cancer after surgical resection. We present the article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-38/rc).
Methods
Study design
This was a single-institution retrospective study of patients who developed local recurrence following surgical resection and were subsequently treated with SBRT. Patients were included in the study if they met the following criteria: (I) biopsy confirmed diagnosis of pancreatic adenocarcinoma, (II) surgical resection of primary disease, (III) local recurrence detected on imaging, (IV) no prior history of radiation therapy, (V) underwent SBRT for local recurrence, (VI) no other local therapy for local recurrence, (VII) routine follow-up with computed tomography (CT) scans. All local recurrences were confirmed by an expert radiologist specializing in gastrointestinal imaging. Common Terminology Criteria for Adverse Events version 4.0 was used for toxicity assessment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Johns Hopkins University (IRB00285919) and individual consent for this retrospective analysis was waived.
SBRT details
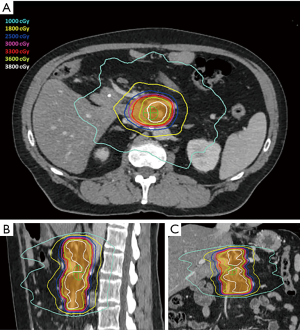
Prior to simulation, patients were considered for endoscopic ultrasound-guided placement of metal fiducials for image-guided radiation therapy. If present, other structures such as surgical clips placed at time of initial surgery, vessel calcifications, or biliary stents were assessed for candidacy for image guidance and if sufficient, fiducial placement was deferred. During simulation, patients were immobilized placed in a Vac-lok device (CIVCO Medical Solutions, Coralville, IA, USA) with arms above their head. Thin-sliced (2 mm) CT scans with intravenous contrast were acquired for treatment planning. To minimize intrafractional motion, patients were simulated under breath-hold conditions (ABC, Elekta, Stockholm, Sweden). Patients unable to tolerate breath-hold underwent a free breathing 4-dimension CT scan. An internal target volume (ITV) was created from the peak expiratory and inspiratory phases of the scan. Pinnacle Treatment System (Phillips Radiation Oncology Systems, Fitchburg, WI, USA) was used for target and organ at risk delineation. The gross tumor volume (GTV) included gross disease plus the full extent of involved and adjacent vasculature. The planning target volume (PTV) was generated by a 3–5 mm isotropic expansion on the GTV or ITV. The following planning objectives were utilized: (I) dose coverage—prescription isodose to cover ≥98% of GTV and ≥90% of PTV, (II) gastrointestinal structures—V33 Gy <1 cc, V25 Gy <20 cc, maximum dose (Dmax) to planning organ at risk volume (3 mm expansion of gastrointestinal structures)—40 Gy, (III) combined kidneys—V12 Gy <25%, (IV) liver—V12 Gy <50% and greater than 700 cc <15 Gy, (V) spinal canal—V8 Gy <1 cc. Pre-treatment and intrafraction cone beam CT scans were acquired for setup evaluation. Shifts were made to align to spine and then to align to fiducials, surgical clips, vessel calcifications, and/or biliary stent. Treatments were performed on an Elekta linear accelerator unit (Elekta). Figure 1 shows a representative SBRT plan. The decision to administer chemotherapy prior to and/or after SBRT was determined by the medical oncology.
Clinical outcome definitions
All clinical outcomes were calculated from completion of SBRT. OS was defined as time to death or last known follow-up for patients who were alive. Local progression included both in-field and out-of-field progression. In-field progression was defined as progression confined to the PTV, while out-of-field progression was defined as progression outside the PTV but within elective nodal areas. For all tumors, elective nodal areas included celiac axis, porta hepatis, superior mesenteric, and peri-aortic lymphatics. Local progression-free survival (LPFS) was defined as time to development of local progression on imaging or last known negative imaging for those without local recurrence. Distant metastasis-free survival (DMFS) was defined as time to development of distant progression on imaging or last known negative imaging for those without distant progression. Progression-free survival (PFS) was defined as time to death, development of any progression on imaging, or last known negative imaging for those without any disease progression.
Statistical analysis
Patient, disease, and treatment information were recorded including age, sex, performance status, disease extent, chemotherapy type and duration, surgery type, tumor size, nodal status, disease grade, local recurrence extent, and SBRT treatment details. Kaplan-Meier analysis was used for survival outcomes including OS, LPFS, DMFS, and PFS. Univariate (UVA) Cox analysis was carried out to identify variables associated with clinical outcomes. Statistical significance was defined as a two-sided P value <0.05. Receiver operating characteristic analysis was used to identify optimal cut-off values for continuous variables significant on UVA. Statistical analyses were performed with JMP version 14.0 (SAS institute, Cary, NC, USA) and SPSS version 27 (SPSS Inc., Chicago, IL, USA).
Results
Patient, primary disease, and primary treatment characteristics
From September 2012 to November 2018, a total of 19 patients were treated with SBRT for locally recurrent pancreatic cancer following surgical resection. Baseline patient, primary disease, and primary treatment characteristics are shown in Table 1. The median age of the cohort was 67.2 years (range, 40.9–89.6 years). At time of initial diagnosis, 16 patients (84.2%) had resectable disease and 3 patients (15.8%) had borderline resectable disease. Median cancer antigen 19-9 (CA 19-9) at initial diagnosis was 49.9 U/mL (range, 1.0–>10,000 U/mL). Neoadjuvant chemotherapy was administered to 5 patients (26.3%), while adjuvant chemotherapy was given to 15 patients (78.9%). Initial surgical resection consisted of Whipple procedure in 14 patients (73.7%), distal pancreatectomy in 3 patients (15.8%), and total pancreatectomy in 2 patients (10.5%). Positive margins were seen in 4 patients (21.1%), and node positive disease was seen in 8 patients (42.1%).
Table 1
| Characteristics | N (%) or median (range) |
|---|---|
| No. of patients | 19 |
| Age (years) | 67.2 (40.9–89.6) |
| Sex | |
| Male | 11 (57.9) |
| Female | 8 (42.1) |
| ECOG | |
| 0 | 8 (42.1) |
| 1 | 8 (42.1) |
| 2 | 3 (15.8) |
| Disease extent | |
| Resectable | 16 (84.2) |
| Borderline resectable | 3 (15.8) |
| Baseline CA 19-9 (U/mL) | 49.9 (1.0–10,000) |
| Neoadjuvant chemotherapy | 5 (26.3) |
| Duration (months) | 4 (1.5–5.0) |
| Regimen | |
| FFX | 2 |
| GnP | 2 |
| FOLFOX plus bevacizumab | 1 |
| Adjuvant chemotherapy | 15 (78.9) |
| Duration (months) | 3.0 (2.0–6.0) |
| Regimen | |
| FFX | 2 |
| GnP | 2 |
| FFX and GnP | 1 |
| Gemcitabine | 6 |
| Gemcitabine plus capecitabine | 4 |
| Surgery type | |
| Whipple procedure | 14 (73.7) |
| Distal pancreatectomy | 3 (15.8) |
| Total pancreatectomy | 2 (10.5) |
| Primary tumor size (cm) | 2.9 (1.0–5.8) |
| Positive margins | 4 (21.1) |
| Node positive | 8 (42.1) |
| Disease grade | |
| II | 13 (68.4) |
| III | 6 (31.6) |
ECOG, Eastern Cooperative Oncology Group; CA 19-9, cancer antigen 19-9; FFX, FOLFIRINOX; GnP, gemcitabine plus nab-paclitaxel.
Local recurrence and treatment information
Table 2 displays local recurrence and treatment information for all patients. The median time from surgical resection to development of local recurrence was 13.5 months (range, 2.3–53.0 months). Extent of vascular involvement was as follows: superior mesenteric vein (9/19, 47.4%), portal vein (8/19, 42.1%), superior mesenteric artery (6/19, 31.6%), and celiac axis (5/19, 26.3%). Peri-SBRT chemotherapy was administered to 10 patients, in the pre-SBRT (3/19, 15.8%) or post-SBRT (8/19, 42.1%) setting for micrometastatic disease and/or to delay recurrence. Pre-SBRT chemotherapy regimens included FOLFIRINOX (FFX), gemcitabine plus nab-paclitaxel, gemcitabine plus capecitabine, and gemcitabine alone for a median of 3 months (range, 2–4 months). Post-SBRT chemotherapy regimens consisted of FFX, gemcitabine, FOLFOX, and capecitabine for a median of 1.5 months (range, 0.5–4 months). Peri-SBRT chemotherapy was not administered to 9 patients due to poor tolerability of initial chemotherapy (n=5), old age (n=1), local progression while on adjuvant chemotherapy (n=1), indolent disease (n=1), and normalization of CA 19-9 after SBRT (n=1). Radiation dose/fractionation regimens were as follows: 33 Gy in 5 fractions (12/19, 63.1%), 25 Gy in 5 fractions (4/19, 21.0%), 28 Gy in 5 fractions (1/19, 5.3%), 27 Gy in 4 fractions (1/19, 5.3%), and 26.4 Gy in 4 fractions (1/19, 5.3%). The median biologically effective dose (BED10) was 54.8 Gy (range, 37.5–54.8 Gy). Eight patients (42.1%) had endoscopic placement of metal fiducials for image-guided radiation therapy. Median PTV was 93.7 cc (range, 17.9–336.8 cc), and median prescription isodose was 85.1% (range, 71.8–97.0%). Radiation modality was intensity modulated radiation therapy in 12 patients (63.2 %) and volumetric modulated arc therapy in 7 patients (36.8%). Active breathing control was used in 14 patients (73.7%), while 5 patients (26.3%) were treated under free-breathing conditions.
Table 2
| Patient no. | Time from surgery to local recurrence (months) | Lesion location | Pre-SBRT chemotherapy (regimen, months) | SBRT dose (Gy)/Fx | BED10 (Gy) | Post-SBRT chemotherapy (regimen, months) | PTV (cc) | Prescription isodose (%) | Modality | Alignment | Breathing control | Pattern of failure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16.9 | SMA, PV | – | 33/5 | 54.8 | Gemcitabine, 1 | 336.8 | 82.0 | VMAT | Surgical clips | Breath hold | DM |
| 2 | 13.6 | PV | – | 33/5 | 54.8 | – | 201.1 | 83.0 | VMAT | Fiducials | Breath hold | – |
| 3 | 15.1 | PV | – | 33/5 | 54.8 | FFX, 4 | 182.7 | 86.0 | VMAT | Surgical clips | Breath hold | LF |
| 4 | 13.4 | PV | – | 33/5 | 54.8 | Capecitabine, 1.5 | 36.7 | 90.0 | VMAT | Fiducials | Breath hold | DM |
| 5 | 16.0 | CA, SMA | – | 33/5 | 54.8 | – | 155.6 | 85.1 | VMAT | Surgical clips | Breath hold | – |
| 6 | 9.9 | SMA | – | 33/5 | 54.8 | – | 57.4 | 88.0 | IMRT | Surgical clips | Breath hold | DM + LF |
| 7 | 1.9 | SMV | – | 28/5 | 43.7 | FFX, 4 | 115 | 83.5 | IMRT | Surgical clips | Breath hold | DM + LF |
| 8 | 1.6 | PV, SMV | GnP, 4 | 33/5 | 54.8 | GnP, 1 | 50.1 | 84.8 | IMRT | Surgical clips | Breath hold | DM + LF |
| 9 | 20.6 | CA | Gemcitabine, 2 | 33/5 | 54.8 | Gemcitabine, 1 | 44.7 | 79.0 | IMRT | Fiducials | Free breathing | DM + LF |
| 10 | 3.0 | CA | – | 33/5 | 54.8 | FFX, 2 | 93.7 | 83.5 | IMRT | Surgical clips | Breath hold | DM |
| 11 | 4.2 | CA | – | 33/5 | 54.8 | FFX, 2 | 115.8 | 92.0 | IMRT | Fiducials | Breath hold | DM |
| 12 | 1.0 | SMA, SMV | – | 25/5 | 37.5 | Unknown | 102.0 | 71.8 | IMRT | Fiducials | Breath hold | DM |
| 13 | 19.5 | SMV | – | 33/5 | 54.8 | – | 132.2 | 81.0 | VMAT | Surgical clips | Free breathing | LF |
| 14 | 14.7 | PV, SMV | FFX, 3 | 26.4/4 | 43.8 | – | 102.3 | 90.0 | IMRT | Aortic calcifications | Free breathing | – |
| 15 | 6.5 | SMA, SMV | – | 25/5 | 37.5 | – | 17.9 | 89.0 | IMRT | Fiducials | Breath hold | DM + LF |
| 16 | 20.1 | SMV | – | 25/5 | 37.5 | – | 79.5 | 97.0 | IMRT | Fiducials | Free breathing | DM + LF |
| 17 | 8.1 | PV, SMV | – | 25/5 | 37.5 | – | 90.1 | 93.5 | IMRT | Biliary stent | Free breathing | – |
| 18 | 53.0 | PV, SMV | – | 33/5 | 54.8 | – | 60.2 | 83.0 | IMRT | Surgical clips | Breath hold | DM |
| 19 | 22.4 | SMA, CA | – | 28/4 | 47.6 | – | 48.5 | 88.2 | VMAT | Fiducials | Breath hold | DM + LF |
SBRT, stereotactic body radiation therapy; BED10, biologically effective dose; PTV, planning target volume; SMA, superior mesenteric artery; PV, portal vein; VMAT, volumetric modulated arc therapy; DM, distant metastasis; FFX, FOLFIRINOX; LF, local failure; CA, celiac axis; IMRT, intensity modulated radiation therapy; SMV, superior mesenteric vein; GnP, gemcitabine plus nab-paclitaxel.
Clinical outcomes
Median follow-up after SBRT was 16.6 months (range, 1.5–87.3 months). Pattern of first failure after SBRT were as follows: distant in 7 patients (46.7%), local in 5 patients (33.3%), and synchronous distant and local in 3 patients (20%). Of the 7 patients who failed distally first, 1 eventually developed local failure. Of the 5 patients who failed locally first, 2 went on to develop distant progression.
Median LPFS was 22.2 months, with 6-month, 1- and 2-year LPFS rates of 86.9%, 63.2%, and 42.1%, respectively (Figure 2A). A total of 9 patients (47.4%) developed local failure after SBRT. Of note, 3 (33.3%) of these local failures were out-of-field, involving the porta hepatis (n=1), pancreaticojejunostomy (n=1), and pancreatic remnant (n=1). On UVA, only BED10 as both a continuous and discrete variable (54.8 vs. <54.8 Gy) was associated with LPFS [hazard ratio (HR) =0.13; 95% CI: 0.02–0.76; P=0.023] (Table 3). Patients treated with BED10 of 54.8 Gy had a median LPFS of 25.0 months (6-month, 1- and 2-year rates of 91.7%, 80.2%, and 64.2%) vs. 7.8 months (6-month, 1- and 2-year rates of 75.0%, 25.0%, and 0%) in patients treated with BED10 <54.8 Gy (log-rank P=0.009) (Figure 2B).
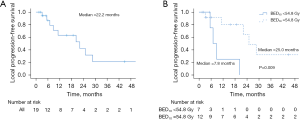
Table 3
| Variable | UVA | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Age (years) | 0.97 | 0.91–1.05 | 0.455 |
| Sex (male vs. female) | 0.40 | 0.10–1.51 | 0.176 |
| BED10 (Gy) | 0.82 | 0.68–0.94 | 0.013 |
| BED10 (54.8 vs. <54.8 Gy) | 0.13 | 0.02–0.76 | 0.023 |
| GTV (cc) | 0.99 | 0.97–1.00 | 0.156 |
| CA 19-9 prior to SBRT (U/mL) | 1.00 | 0.99–1.00 | 0.863 |
| Interval from surgery to local recurrence (months) | 0.98 | 0.91–1.04 | 0.621 |
| Peri-SBRT chemotherapy (yes vs. no) | 0.73 | 0.19–2.74 | 0.637 |
UVA, univariate; LPFS, local progression-free survival; HR, hazard ratio; BED10, biologically effective dose; GTV, gross tumor volume; CA 19-9, cancer antigen 19-9; SBRT, stereotactic body radiation therapy.
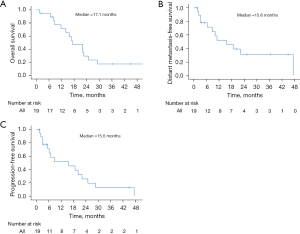
Median OS was 17.1 months, with 6-month, 1- and 2-year OS rates of 94.7%, 71.3%, and 29.7%, respectively (Figure 3A). At last follow-up, 14 patients were dead and 5 were alive or had unknown status. Median DMFS was 15.6 months, with 6-month, 1- and 2-year DMFS rates of 77.8%, 51.9%, and 31.1%, respectively (Figure 3B). Median PFS was 15.6 months, with 6-month, 1- and 2-year PFS rates of 71.3%, 51.9%, and 25.9%, respectively (Figure 3C). At last follow-up, 4 patients (21.1%) were without any evidence of disease. Tables S1-S3 demonstrate UVA for other clinical outcomes. No variables were associated with OS, DMFS, or PFS, with the exception of age (HR =0.99; 95% CI: 0.88–0.99; P=0.035) for DMFS.
Radiation related toxicity
Acute grade 1–2 toxicity was seen in 15 patients (78.9%), with the most common being fatigue (n=12), nausea (n=7), pain (n=3), anorexia (n=2), and diarrhea (n=1). There were no acute grade ≥3 toxicity events. Only one event of late grade 3 toxicity was observed. One patient (5.3%) developed gastric perforation approximately 13 months after being treated with SBRT to 33 Gy in 5 fractions. Abdominal CT showed fluid and air collections posterior to the gastric wall adjacent to prior placed surgical clips. The patient was admitted to an outside institution so details regarding intervention is unknown. However, the patient recovered and had follow-up roughly 1 month after this event. Stomach Dmax was 30.9 Gy. There were no cases of grade 4+ toxicity.
Discussion
We demonstrate that SBRT for locally recurrent pancreatic cancer after surgical resection to a median dose of 33 Gy in five fractions (BED10 of 54.8 Gy) is well tolerated. There were no cases of acute grade ≥3 toxicity, 1 case (5.3%) of late grade 3 toxicity, and no grade 4–5 events. Durable local control for the overall cohort was modest, with 6-month, 1- and 2-year LPFS rates after SBRT of 86.9%, 63.2%, and 42.1%, respectively, but local failure was particularly poor for patients treated with a BED10 of <54.8 Gy. Out-of-field local failures were also seen. Both findings highlight the need to further optimize both dose and treatment volumes in the locally recurrent setting.
Pancreatic cancer is thought to be a systemic disease with distant failure being most common after primary treatment (5,6,21). In fact, distant progression is seen in up to 79% of patients after curative resection (21). However, locoregional recurrence is also problematic (22). A secondary analysis of the ESPAC-4 phase III trial and an Australian phase II study demonstrated that the pattern of first failure after surgical resection was locoregional in 49.7% and 45.5% of patients, respectively (7,8). Given improvements in systemic therapy, with multi-agent chemotherapy, it is likely that the incidence of local failures will rise (11,12). Furthermore, uncontrolled locoregional disease can be severely debilitating and can negatively impact mortality. Cardillo et al. (9) showed that the majority of hospitalizations in pancreatic cancer patients were a result of local progression leading to complications such as cholangitis, biliary obstruction, and gastrointestinal bleeding. Additionally, an autopsy study showed that up 30% of pancreatic cancer patients die from locally destructive disease (10). Therefore, durable locoregional control is imperative in the management of pancreatic cancer.
Locoregional disease after curative resection can be managed with re-resection, radiation therapy, chemotherapy, or combination therapy. The management approach depends on many factors including performance status, disease extent, and patient preference. Recent studies have shown that re-resection can lead to promising outcomes (13-15,23). Miyazaki et al. (15) showed that re-resection of locally recurrent disease after initial pancreatectomy was associated with improved median OS (25.0 vs. 9.3 months, P<0.01). Strobel et al. (14) demonstrated similar findings (median OS, 26.0 vs. 10.8 months, P<0.05), with further improvement in OS with R0 resection. However, perioperative morbidity rates can be as high as 27% (15). Additionally, many patients are not candidates for surgery due to medical co-morbidities and/or too locally advanced disease characterized by extensive vascular involvement at time of recurrence. As such, minimally invasive local therapies such as SBRT may have a role in these situations.
There are several reports on the role of SBRT for locally recurrent pancreatic cancer after resection (16-20). However, many of these studies are limited by heterogeneous patient populations, such as including patients with unresected disease and/or those with prior radiation. The two largest studies are by Zeng et al. and a prior analysis from our institution by Ryan et al. (16,17). Zeng et al. (17) reported on 24 radiation-naïve patients who developed local recurrence after surgical resection and were subsequently treated with SBRT. They demonstrated excellent 6-month and 1-year local control rates of 95.2% and 83.8%, respectively (16). Ryan et al. (16) reported on a similar cohort of 51 patients, of which 26 were radiation-naïve and 25 had prior radiation. The majority (88%) of patients in their study received pre-SBRT chemotherapy compared to just 3 patients (15.8%) in our cohort. In the 26 radiation-naïve patients, 6-month and 1-year local control rates were 75% and 62%, respectively, which is consistent with our findings (6-month: 86.9%; 1-year: 63.2%). Improved local control in the series by Zeng et al. (17) may be explained by the use of higher radiation dose. In their study, the median SBRT dose/fractionation was 45 Gy/5 fractions (BED10 of 85.5 Gy), compared to 33 Gy/5 fractions (BED10 of 54.8 Gy) in our study and 25 Gy/5 fractions (BED10 of 37.5 Gy) in the study by Ryan et al. (16). Indeed, in our cohort, we demonstrate that low BED10 (<54.8 Gy) was associated with significantly inferior local control. These findings may highlight the importance of higher doses for durable local control. Dose escalation for local recurrence after surgery may be an attractive approach given recent data demonstrating promising local control and OS outcomes for unresectable disease treated with higher doses (24,25). Additionally, dose escalation in the post-Whipple setting may be better tolerated than expected given that the duodenum has been removed. Further studies investigating the feasibility and efficacy of ablative radiation for local recurrences post-surgery are warranted.
Moreover, in our study, some patients developed out-of-field locoregional failures. Similar findings were demonstrated by Zeng et al. (17). Although they only included in-field failures in their analysis of LPFS, 50% of all locoregional failures were out-of-field. These data suggest that treatment of the gross disease alone, as visualized on cross-sectional imaging, may not be sufficient and that inclusion of elective tissue at risk for microscopic disease may be warranted. Indeed, there is controversy regarding optimal treatment volumes of unresectable pancreatic cancer. Consensus guidelines suggest treating gross disease alone, while others have shown that elective nodal irradiation may be warranted (26-30). Recent studies have shown that elective coverage is well tolerated even when given with ablative doses to gross disease (24,31). Additionally, Miller et al. (30) demonstrated that elective nodal irradiation can improve locoregional control (2-year: 22.6% vs. 44.6%, P=0.021). Unfortunately, there are no studies investigating elective nodal treatment in the locally recurrent setting. Interestingly, two of the out-of-field local failures in our cohort were seen at the pancreaticojejunostomy and the pancreatic remnant, raising the question as to whether these structures should also be considered for inclusion in the radiation field in this setting. Further investigation into appropriate treatment volumes for these patients should be pursued.
Limitations of this retrospective analysis should be recognized. Treatment of the primary disease was heterogeneous with regards to chemotherapy and surgical approach. Similarly, peri-SBRT chemotherapy regimens and durations varied significantly, which may have impacted clinical outcomes. The small sample size also limits the strength of the findings and limited our ability to identify variables associated with outcomes. Such information would have potentially allowed us to comment on ideal candidates for SBRT. The strengths of this study include its long follow-up time, homogenous patient population, and homogenous SBRT dose/fractionation. These findings add to the limited literature on the role of SBRT for isolated local recurrence following resection of pancreatic cancer.
In conclusion, we show that SBRT to a median dose of 33 Gy in five fractions for locally recurrent pancreatic cancer after surgical resection is well tolerated, with just 1 episode of late grade 3 toxicity and no events of grade 4–5 toxicity. Local control was modest with 6-month, 1- and 2-year LPFS rates of 86.9%, 63.2%, and 42.1%, respectively. Of note, radiation dose was significantly associated with local control. As such, further investigation into dose escalation and appropriate treatment volumes for locally recurrent disease after surgical resection is warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-38/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-38/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-38/coif). JMH serves as an unpaid editorial board member of Journal of Gastrointestinal Oncology from January 2021 to December 2022. JMH is a former employee of pancreatic action network and consultant for the 1440 foundation. JMH currently has a grant through the 1440 foundation where funds are provided to Northwell for protected time to lead the Canopy Cancer Collective Learning Health Network. JM receives royalties from Uptodate and Springer and honorarium from Springer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Johns Hopkins University (IRB00285919) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma. Version 2.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019;10:10-27. [Crossref] [PubMed]
- Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer 1990;66:56-61. [Crossref] [PubMed]
- Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009;35:600-4. [Crossref] [PubMed]
- Barbour AP, Samra JS, Haghighi KS, et al. The AGITG GAP Study: A Phase II Study of Perioperative Gemcitabine and Nab-Paclitaxel for Resectable Pancreas Cancer. Ann Surg Oncol 2020;27:2506-15. [Crossref] [PubMed]
- Jones RP, Psarelli EE, Jackson R, et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg 2019;154:1038-48. [Crossref] [PubMed]
- Cardillo N, Seible DM, Fero KE, et al. Clinical Impact of Local Progression in Pancreatic Cancer. J Natl Compr Canc Netw 2018;16:711-7. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Suzuki S, Furukawa T, Oshima N, et al. Original Scientific Reports: Clinicopathological Findings of Remnant Pancreatic Cancers in Survivors Following Curative Resections of Pancreatic Cancers. World J Surg 2016;40:974-81. [Crossref] [PubMed]
- Strobel O, Hartwig W, Hackert T, et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol 2013;20:964-72. [Crossref] [PubMed]
- Miyazaki M, Yoshitomi H, Shimizu H, et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile? Surgery 2014;155:58-66. [Crossref] [PubMed]
- Ryan JF, Groot VP, Rosati LM, et al. Stereotactic Body Radiation Therapy for Isolated Local Recurrence After Surgical Resection of Pancreatic Ductal Adenocarcinoma Appears to be Safe and Effective. Ann Surg Oncol 2018;25:280-9. [Crossref] [PubMed]
- Zeng XL, Wang HH, Meng MB, et al. Stereotactic body radiation therapy for patients with recurrent pancreatic adenocarcinoma at the abdominal lymph nodes or postoperative stump including pancreatic stump and other stump. Onco Targets Ther 2016;9:3985-92. [Crossref] [PubMed]
- Wild AT, Hiniker SM, Chang DT, et al. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: experience from two institutions. J Gastrointest Oncol 2013;4:343-51. [PubMed]
- Dagoglu N, Callery M, Moser J, et al. Stereotactic Body Radiotherapy (SBRT) Reirradiation for Recurrent Pancreas Cancer. J Cancer 2016;7:283-8. [Crossref] [PubMed]
- Sutera P, Bernard ME, Wang H, et al. Stereotactic Body Radiation Therapy for Locally Progressive and Recurrent Pancreatic Cancer after Prior Radiation. Front Oncol 2018;8:52. [Crossref] [PubMed]
- Winter JM, Tang LH, Klimstra DS, et al. Failure patterns in resected pancreas adenocarcinoma: lack of predicted benefit to SMAD4 expression. Ann Surg 2013;258:331-5. [Crossref] [PubMed]
- Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg 2006;10:511-8. [Crossref] [PubMed]
- Boone BA, Zeh HJ, Mock BK, et al. Resection of isolated local and metastatic recurrence in periampullary adenocarcinoma. HPB (Oxford) 2014;16:197-203. [Crossref] [PubMed]
- Reyngold M, O'Reilly EM, Varghese AM, et al. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol 2021;7:735-8. [Crossref] [PubMed]
- Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys 2016;94:755-65. [Crossref] [PubMed]
- Palta M, Godfrey D, Goodman KA, et al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol 2019;9:322-32. [Crossref] [PubMed]
- Oar A, Lee M, Le H, et al. Australasian Gastrointestinal Trials Group (AGITG) and Trans-Tasman Radiation Oncology Group (TROG) Guidelines for Pancreatic Stereotactic Body Radiation Therapy (SBRT). Pract Radiat Oncol 2020;10:e136-46. [Crossref] [PubMed]
- Brunner TB, Haustermans K, Huguet F, et al. ESTRO ACROP guidelines for target volume definition in pancreatic cancer. Radiother Oncol 2021;154:60-9. [Crossref] [PubMed]
- Kharofa J, Mierzwa M, Olowokure O, et al. Pattern of Marginal Local Failure in a Phase II Trial of Neoadjuvant Chemotherapy and Stereotactic Body Radiation Therapy for Resectable and Borderline Resectable Pancreas Cancer. Am J Clin Oncol 2019;42:247-52. [Crossref] [PubMed]
- Miller JA, Toesca DAS, Baclay JRM, et al. Pancreatic Stereotactic Body Radiation Therapy With or Without Hypofractionated Elective Nodal Irradiation. Int J Radiat Oncol Biol Phys 2022;112:131-42. [Crossref] [PubMed]
- Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract Radiat Oncol 2021;11:134-47. [Crossref] [PubMed]


