
Immunotherapy in synchronous MSI-H rectal adenocarcinoma and upper tract urothelial carcinoma: a case report
Introduction
The treatment paradigm for locally advanced rectal cancer is evolving. Though neoadjuvant chemotherapy is becoming more widely adopted, it is associated with low response rates in patients with tumors exhibiting mismatch repair deficiency (dMMR) or microsatellite instability-high (MSI-H) (1). Pembrolizumab is currently approved as first-line therapy for patients with dMMR or MSI-H unresectable or metastatic colorectal cancer, and immunotherapy has already been established for treatment-refractory, advanced dMMR/MSI-H colorectal tumors (1-3). The role of immunotherapy in the neoadjuvant and adjuvant settings, however, remains undefined. We present the following case report, in accordance with the CARE checklist, of a patient with Lynch syndrome with synchronous locally advanced rectal cancer (LARC) and upper tract urothelial carcinoma (UTUC). The patient was initially treated using neoadjuvant chemotherapy and chemoradiation, leading to a partial response. She was subsequently treated with surgical resection followed by adjuvant immunotherapy with nivolumab and ipilimumab, maintaining a durable disease-free interval of nearly 21 months. This case highlights the role of PD-1 and CTL4A inhibitors in patients with dMMR/MSI-H synchronous primary malignancies usually requiring separate treatment strategies. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-81/rc).
Case presentation
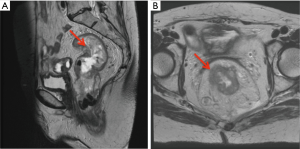
A 62-year-old woman with a family history of Lynch syndrome presented with unintentional weight loss, abdominal pain, and hematochezia. Initial computed tomography (CT) scan showed an infiltrative rectal mass with pelvic lymphadenopathy. She was also found to have a soft tissue lesion at the left ureter causing obstructive hydronephrosis. Subsequent magnetic resonance imaging (MRI) pelvis revealed a 10 cm rectal tumor, extra-mesorectal lymphadenopathy and threatened mesorectal fascia, consistent with T3cN2M0 disease (Figure 1A,1B).
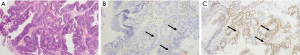
Biopsy of the rectal mass revealed an infiltrative rectal adenocarcinoma with loss of MLH1 and PMS2 expression (Figure 2A-2C). Paired germline and tumor genetic testing revealed a pathogenic mutation in MLH1 c.589-1G>A, consistent with Lynch syndrome. Cystoscopy and ureteroscopy with biopsy of the ureteral mass was non-diagnostic, showing only fragments of stromal tissue. The patient was treated with six cycles of neoadjuvant capecitabine and oxaliplatin followed by five weeks of radiation (approximately 4,500–5,000 cGy) with capecitabine. A pelvic MRI at the end of chemoradiation showed a residual 7.3 cm rectal mass, persistent extra-mesorectal lymphadenopathy and threatened mesorectal fascia (Figure 3A,3B).
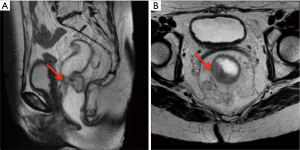
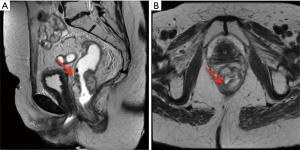
Given these findings, the patient completed three additional cycles of capecitabine and oxaliplatin prior to resection. Repeat pelvic MRI showed that while the rectal tumor had decreased to 4.7 cm, there was continued threatened mesorectal fascia (Figure 4A,4B). The patient subsequently underwent abdominoperineal resection with a left partial nephroureterectomy as her left kidney had become nonfunctional due to obstruction from the ureteral mass. Pathology of the rectosigmoid mass showed an invasive mucinous adenocarcinoma with positive lymph nodes (5 out of 16), consistent with pT3N2a disease. Pathology from the nephroureterectomy revealed high-grade invasive UTUC (0.9 cm in largest diameter), with tumor causing urethral stricture.
Immunohistochemical staining of the urothelial carcinoma again demonstrated loss of MLH1 and PMS2 expression, as previously found in the rectal adenocarcinoma (Figure 5A-5C). There was tumor invasion beyond the muscularis and positive margins, consistent with pT3 disease. Post-surgical imaging by CT scan, however, did not show any evidence of residual disease.

Taking into consideration the high burden of residual disease for rectal cancer, positive margins for UTUC, and MSI-H status, multidisciplinary tumor board recommendation was for adjuvant immunotherapy using nivolumab and ipilimumab. The patient was then treated with four cycles of nivolumab (3 mg/kg) and ipilimumab (1 mg/kg), followed by ten cycles of maintenance nivolumab 240 mg every 2 weeks. The patient eventually discontinued maintenance nivolumab due to arthralgias. Surveillance imaging with CT chest, abdomen and pelvis along with cystoscopy has shown no evidence of disease, which has lasted for nearly 21 months.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Lynch syndrome, or hereditary non-polyposis colorectal cancer syndrome, is an autosomal dominant genetic disorder caused by germline mutations in at least one of the DNA mismatch repair genes MSH2, MLH1, MSH6, or PMS2 (4,5). Patients with Lynch syndrome have increased lifetime risk for malignancies such as colorectal, endometrial, ovarian, pancreatic, and urothelial cancer (4,5). Affected individuals can develop both synchronous and metachronous primary cancers; some case reports note as many as three synchronous and five metachronous primary cancers (6,7). Tumors arising from Lynch syndrome are dMMR, and the majority are MSI-H (4,5). It is estimated that one out of 35 cases of colorectal cancer (CRC) can be attributed to Lynch syndrome (4). The prevalence of dMMR/MSI-H CRC, which includes both cases arising from Lynch syndrome and sporadically, is estimated to be around 10–13% (8).
In LARC, the addition of neoadjuvant chemotherapy has been shown to reduce the risk of distant metastases, increase rates of pathological complete response (pCR), and potentially increase rates of metastasis-free and disease-free survival (DFS) (9-11). However, there is increasing evidence that neoadjuvant chemotherapy may not be as effective for dMMR/MSI-H rectal tumors. In a retrospective case control study, Cercek et al. reported that patients with dMMR/MSI-H rectal tumors treated with neoadjuvant chemotherapy had higher rates of disease progression compared to proficient mismatch repair (pMMR) tumors (29% in dMMR tumors vs. 0% in pMMR tumors, P=0.0001) (1). Updated results from the FOxTROT trial, a randomized Phase III trial examining the addition of neoadjuvant chemotherapy compared to surgery and adjuvant chemotherapy alone in locally advanced colon cancer, found similar results (12). Neoadjuvant chemotherapy for dMMR/MSI-H tumors was associated with decreased DFS and lower response rates, with an estimated 75% of dMMR/MSI-H tumors having no pathologic response to chemotherapy (1,12). This evidence suggests that using neoadjuvant chemotherapy in dMMR/MSI-H rectal or colon tumors may lead to inferior outcomes and a higher risk of recurrence.
Neoadjuvant immunotherapy may serve as a promising alternative to neoadjuvant chemotherapy in the treatment of dMMR/MSI-H colorectal tumors. In the exploratory NICHE study, 21 patients with early-stage dMMR colon cancer received neoadjuvant nivolumab and ipilimumab prior to surgery. Major pathologic response (MPR) was seen in 100% of patients (95% CI: 86–100%), with 12 patients achieving pCR (13). While there have not been large randomized controlled trials exploring the use of neoadjuvant immunotherapy for LARC, there have been several published case reports. Zhang et al. reported two cases in which patients with dMMR LARC received neoadjuvant nivolumab (14). After six cycles of nivolumab, one patient proceeded to surgery and achieved a pCR, while the other had a clinical CR (14). Demisse et al. described the use of neoadjuvant immunotherapy-based systemic treatment in three patients with dMMR LARC in a case series: one patient received single-agent pembrolizumab, one received combination nivolumab and ipilimumab after failing total neoadjuvant therapy, and one received pembrolizumab plus FOLFOX (15). All three patients had a significant response (15). There are several ongoing Phase I and II trials investigating neoadjuvant immunotherapy in combination with chemoradiation (Table 1) (16-20).
Table 1
| Name | National clinical trial number | Phase | Staging criteria | Immunotherapy | Design |
|---|---|---|---|---|---|
| Safety and Feasibility of PD-1 Blockade in the Treatment of Rectal Cancer (16) | NCT04357587 | I | Stage II, stage III, or locally advanced stage IV rectal adenocarcinoma | Pembrolizumab | Neoadjuvant pembrolizumab combined with external beam radiation and capecitabine |
| Safety and Efficacy of Atezolizumab Combined to Preoperative Radio-chemotherapy in Localized Rectal Cancer (R-IMMUNE) (17) | NCT03127007 | Ib/II | Stage II or stage III rectal adenocarcinoma | Atezolizumab | Neoadjuvant atezolizumab with 5-fluorouracil and radiotherapy compared to 5-fluorouracil (5-FU) and radiotherapy alone |
| Immunotherapy in Locally Advanced Rectal Cancer (AVANA) (18) | NCT03854799 | II | Node positive, cT3a, or cT4 rectal adenocarcinoma | Avelumab | Neoadjuvant avelumab combined with external beam radiation and capecitabine |
| Study of Induction PD-1 Blockade in Subjects With Locally Advanced Mismatch Repair Deficient Solid Tumors (19) | NCT04165772 | II | Stage II or stage III MSI-H/dMMR rectal adenocarcinoma | Dostarlimab | Up to six months of treatment with dostarlimab. If CR not achieved, treatment with capecitabine or 5-FU combined with radiation will follow. If CR again not achieved, surgical resection or standard of care therapy will follow |
| INNATE: Immunotherapy During Neoadjuvant Therapy for Rectal Cancer (20) | NCT04130854 | II | Stage II or stage III rectal adenocarcinoma with high risk featuresb; stage IV rectal adenocarcinoma with liver-limited diseasec | APX005M (anti-CD40 agonist) | Neoadjuvant APX005M with mFOLFOX and radiation versus mFOLFOX and radiation alone |
a, high-risk defined as tumor extending to within 1 mm of mesorectal fascia, lower third (≤6 cm from anal verge), or tumor extending ≥5 mm into perirectal fat; b, high-risk features defined as: distal (<1 cm from anal ring), bulky cT4 or within 3 mm of MR fascia, extramural venous invasion, not candidate for sphincter preservation; c, liver-limited disease must have ≤3 lesions.
UTUC is a rare malignancy, accounting for 2–5% of urothelial tumors (21). The current standard of care is surgical resection via nephroureterectomy with bladder cuff removal or distal ureterectomy combined with regional lymphadenectomy in patients with high grade tumors, followed by adjuvant chemotherapy (22). The role of adjuvant chemotherapy was established in the POUT trial, a Phase III randomized controlled trial comparing adjuvant chemotherapy (platinum plus gemcitabine) vs. surveillance after nephroureterectomy in patients with pT2-T4, N0-N3 M0 or pTany N1-3 M0 UTUC (23). The trial found a significant improvement in DFS (HR 0.45, 95% CI: 0.30–0.68, P=0.0001) and 3-year event free survival (71%, 95% CI: 61–78% for chemotherapy group vs. 46%, 95% CI: 36–56% for surveillance group) (23). Without adjuvant chemotherapy, approximately one-third of patients recur and the 5-year survival rate is less than 50% (24,25). Neoadjuvant chemotherapy with cisplatin-based therapies has been effective in inducing pathological downstaging—the strongest prognostic factor for recurrence-free, cancer-specific and overall survival (24). However, some patients are not eligible for these chemotherapies owing to poor renal function and/or functional status (26).
Similar to LARC, the data for neoadjuvant immunotherapy in UTUC is limited but appears promising (26). Ikarashi et al. described a patient with UTUC who received neoadjuvant pembrolizumab after failing chemotherapy and subsequently achieved sustained complete remission (25). The NABUCCO trial examined the use of 2–3 cycles of neoadjuvant nivolumab and ipilimumab in 24 patients with locoregionally advanced urothelial cancer, including one patient with upper tract involvement (27). Eleven patients achieved a pCR and two achieved a MPR, defined as <10% of tumor cells seen in the tumor bed (27). The data supporting adjuvant immunotherapy in UTUC, however, have not yet been fully reported. Currently there are three ongoing phase III studies (IMVIGOR010, CheckMate-274, AMBASSADOR) investigating the role of immunotherapy in high-risk muscle invasive urothelial cancer (28-30). Preliminary data from CheckMate-274, a Phase III trial evaluating adjuvant nivolumab in high-risk muscle-invasive urothelial carcinoma compared to placebo, have shown that adjuvant nivolumab improves DFS (though the number of UTUC patients included in this trial is not reported) (29).
As discussed above, neoadjuvant immunotherapy may eventually play a significant role in treating patients with dMMR/MSI-H LARC. The evidence suggests that neoadjuvant chemotherapy is inferior in patients with dMMR/MSI-H LARC compared to patients with pMMR tumors (1). Case reports have shown neoadjuvant immunotherapy as a promising alternative; however, further investigation is needed to support its routine use (14,15). Furthermore, in patients with two or more synchronous primaries (such as in Lynch syndrome), neoadjuvant or adjuvant immunotherapy can be considered as a potential option.
Conclusions
Here we present a patient with Lynch Syndrome who presented with a locally advanced, dMMR/MSI-H rectal adenocarcinoma and was also found to have a synchronous UTUC. She was initially treated with neoadjuvant chemotherapy and chemoradiation, achieving a clinical partial response. She then underwent surgery and was considered at high risk for recurrence, thus receiving adjuvant immunotherapy with nivolumab and ipilimumab based on her dMMR/MSI-H LARC and high-grade UTUC. She remained without evidence of recurrence for nearly 21 months. This case report highlights the importance of identifying dMMR/MSI-H status in LARC, and reviews the role of immunotherapies such as PD-1 and CTLA-4 inhibitors in both LARC and non-metastatic UTUC. This case also underscores the potential role of adjuvant immunotherapy as an alternative to chemotherapy in patients with synchronous primary cancers. Strategies utilizing neoadjuvant and adjuvant immunotherapy should be investigated further in localized CRC, particularly in the setting of dMMR/MSI-H status, where the evidence is growing to support a diminished effect of conventional neoadjuvant and/or adjuvant chemotherapy in these subsets.
Acknowledgments
The authors greatly appreciate Dr. Simran Sehkon of the UC Davis Department of Radiology for assistance with image selection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-81/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-81/coif). Dr. MP has served on the advisory board for AstraZeneca (AZ) to discuss treatments for urothelial cancer, including the use of immune checkpoint inhibitors. No content of this manuscript was affected by this, and in fact this advisory board was held after this manuscript was written. Dr. EK has consulted for Eisai and Celgene; served on the speaker bureau for Eisai; and participated on the advisory and data safety monitoring board for Lilly and Taiho, respectively. Dr. JG has received funding and served as a consultant for EMD Serono, Elsevier, Exelixis, QED Therapeutics, Natera, Basilea, HalioDx, Eisai, and Janssen. Dr. WH has received funding from Intuitive Surgical in the form of two paid dinners. Dr. MC has served as a consultant for Bristol Myers Squibb (BMS), Genentech, and AZ; served as part of the speaker bureau for BMS’s gastric carcinoma program; and participated in the data safety monitoring board/advisory board for Sea-Gen, BMS, AZ, and Genentech. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cercek A, Dos Santos Fernandes G, Roxburgh CS, et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin Cancer Res 2020;26:3271-9. [Crossref] [PubMed]
- FDA. FDA approves pembrolizumab for first-line treatment of MSI-H/dMMR colorectal cancer. [cited 2020 Nov 12]. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-first-line-treatment-msi-hdmmr-colorectal-cancer
- Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019;16:361-75. [Crossref] [PubMed]
- Bhattacharya P, McHugh TW. Lynch Syndrome. Treasure Island (FL): StatPearls Publishing; 2022 Jan.
- Duraturo F, Liccardo R, De Rosa M, et al. Genetics, diagnosis and treatment of Lynch syndrome: Old lessons and current challenges. Oncol Lett 2019;17:3048-54. [Crossref] [PubMed]
- Mendez LE, Atlass J. Triple synchronous primary malignancies of the colon, endometrium and kidney in a patient with Lynch syndrome treated via minimally invasive techniques. Gynecol Oncol Rep 2016;17:29-32. [Crossref] [PubMed]
- Hu H, Li H, Jiao F, et al. Association of a novel point mutation in MSH2 gene with familial multiple primary cancers. J Hematol Oncol 2017;10:158. [Crossref] [PubMed]
- Lorenzi M, Amonkar M, Zhang J, et al. Epidemiology of Microsatellite Instability High (MSI-H) and Deficient Mismatch Repair (dMMR) in Solid Tumors: A Structured Literature Review. J Oncol 2020; [Crossref]
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702-15. [Crossref] [PubMed]
- Hospers G, Bahadoer RR, Dijkstra EA, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. J Clin Oncol 2020;38:4006. [Crossref]
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152-60. [Crossref] [PubMed]
- Seligmann JF. FOxTROT Collaborative Group. FOxTROT: neoadjuvant FOLFOX chemotherapy with or without panitumumab (Pan) for patients (pts) with locally advanced colon cancer (CC). J Clin Oncol 2020;38:4013. [Crossref]
- Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566-76. [Crossref] [PubMed]
- Zhang J, Cai J, Deng Y, et al. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology 2019;8:e1663108. [Crossref] [PubMed]
- Demisse R, Damle N, Kim E, et al. Neoadjuvant Immunotherapy-Based Systemic Treatment in MMR-Deficient or MSI-High Rectal Cancer: Case Series. J Natl Compr Canc Netw 2020;18:798-804. [Crossref] [PubMed]
- ClinicalTrials.gov. Safety and Feasibility of PD-1 Blockade in the Treatment of Rectal Cancer. [cited 2020 Nov 12]. Available online: https://clinicaltrials.gov/ct2/show/NCT04357587
- ClinicalTrials.gov. Safety and Efficacy of Atezolizumab Combined to Preoperative Radio-chemotherapy in Localized Rectal Cancer (R-IMMUNE). [cited 2020 Nov 12]. Available online: https://clinicaltrials.gov/ct2/show/NCT03127007
- ClinicalTrials.gov. Immunotherapy In Locally Advanced Rectal Cancer (AVANA). [cited 2020 Nov 12]. Available online: https://clinicaltrials.gov/ct2/show/NCT03854799
- ClinicalTrials.gov. Study of Induction PD-1 Blockade in Subjects With Locally Advanced Mismatch Repair Deficient Solid Tumors. [cited 2020 Nov 12]. Available online: https://clinicaltrials.gov/ct2/show/NCT04165772
- ClinicalTrials.gov. INNATE: Immunotherapy During Neoadjuvant Therapy for Rectal Cancer. [cited 2020 Nov 12]. Available online: https://clinicaltrials.gov/ct2/show/NCT04130854
- Golan S, Nadu A, Lifshitz D. The role of diagnostic ureteroscopy in the era of computed tomography urography. BMC Urol 2015;15:74. [Crossref] [PubMed]
- Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:329-54. [Crossref] [PubMed]
- Birtle AJ, Chester JD, Jones RJ, et al. Results of POUT: A phase III randomised trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J Clin Oncol 2018;36:407. [Crossref]
- Foerster B, Abufaraj M, Petros F, et al. Efficacy of Preoperative Chemotherapy for High Risk Upper Tract Urothelial Carcinoma. J Urol 2020;203:1101-8. [Crossref] [PubMed]
- Ikarashi D, Kitano S, Ishida K, et al. Complete Pathological Response to Neoadjuvant Pembrolizumab in a Patient With Chemoresistant Upper Urinary Tract Urothelial Carcinoma: A Case Report. Front Oncol 2020;10:564714. [Crossref] [PubMed]
- Teo MY, Rosenberg JE. Perioperative Immunotherapy in Muscle-Invasive Bladder Cancer and Upper Tract Urothelial Carcinoma. Urol Clin North Am 2018;45:287-95. [Crossref] [PubMed]
- van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med 2020;26:1839-44. [Crossref] [PubMed]
- Hussain MHA, Powles T, Albers P, et al. IMvigor010: Primary analysis from a phase III randomized study of adjuvant atezolizumab (atezo) versus observation (obs) in high-risk muscle-invasive urothelial carcinoma (MIUC). J Clin Oncol 2020;38:5000. [Crossref]
- Bristol Myers Squibb. Opdivo (nivolumab) Significantly Improves Disease Free-Survival vs. Placebo as Adjuvant Therapy for Patients with High-Risk, Muscle-Invasive Urothelial Carcinoma in Phase 3 CheckMate -274 Trial. [cited 2020 Nov 16]. Available online: https://news.bms.com/news/details/2020/Opdivo-nivolumab-Significantly-Improves-Disease-Free-Survival-vs.-Placebo-as-Adjuvant-Therapy-for-Patients-with-High-Risk-Muscle-Invasive-Urothelial-Carcinoma-in-Phase-3-CheckMate--274-Trial/default.aspx
- ClinicalTrials.gov. Testing MK-3475 (Pembrolizumab) After Surgery for Localized Muscle-Invasive Bladder Cancer and Locally Advanced Urothelial Cancer (AMBASSADOR). [cited 2020 Nov 17]. Available online: https://clinicaltrials.gov/ct2/show/NCT03244384?term=adjuvant&cond=Urothelial+Carcinoma+Ureter&draw=2&rank=2


