Dose modification and efficacy of nab-paclitaxel plus gemcitabine vs. gemcitabine for patients with metastatic pancreatic cancer: phase III MPACT trial
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States and Europe (1,2). Surgical resection is the only curative approach for pancreatic cancer (3), but because symptoms are subtle or absent in early stages, the large majority of patients present with advanced nonresectable disease (1). Historically, the prognosis for patients with metastatic disease has been particularly poor (1). For example, treatment with the long-time standard of care, gemcitabine (Gem) monotherapy, led to median overall survival (OS) values of approximately 5 to 7 months in multiple phase III trials of patients with metastatic pancreatic cancer (4-16). However, two phase III trials have demonstrated clinically meaningful survival advantages for an experimental regimen over Gem monotherapy: the PRODIGE ACCORD 11 trial of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) and the MPACT trial of nab-paclitaxel (nab-P) + Gem (17,18). To avoid toxicity with the per protocol FOLFIRINOX regimen, investigators have experimented with modification of starting components and doses; studies of modified FOLFIRINOX have demonstrated inconsistent efficacy results (19,20). The tolerability profile of nab-P + Gem allowed most doses of nab-P (71%) to be delivered at the per protocol level in the MPACT trial (18). However, little is known about how dose modifications may have affected treatment outcomes.
In the MPACT trial, nab-P + Gem demonstrated a longer OS [median, 8.5 vs. 6.7 months; hazard ratio (HR), 0.72; 95% CI, 0.62–0.83; P<0.001], a longer progression-free survival (PFS) (median, 5.5 vs. 3.7 months; HR, 0.69; 95% CI, 0.58–0.82; P<0.001) and a higher overall response rate (ORR) [23% vs. 7%; response rate ratio (RRR) 3.19; 95% CI, 2.18–4.66; P<0.001] than Gem alone (18). Furthermore, the OS benefit for nab-P + Gem over Gem alone was consistent across patient subgroups, and an updated analysis revealed a >2-month difference in OS at the median (8.7 vs. 6.6 months; HR, 0.72; 95% CI, 0.62–0.83; P<0.001) (21). Long-term survivors (≥3 years) were also observed in the updated OS analysis, but only in the nab-P + Gem arm (4%). Treatment-related grade ≥3 neutropenia, leukopenia, peripheral neuropathy (no grade 4), and fatigue occurred more often in the nab-P + Gem arm than in the Gem-alone arm (18). These toxicities were effectively managed with dose reductions and delays.
Dose reductions and delays are standard methods to reduce the toxic effects of chemotherapy, particularly myelosuppressive chemotherapy. A correlation has been noted between dose intensity and efficacy in some clinical trials (22,23). However, in ovarian cancer and non-small cell lung cancer, dose modifications of specific treatments had no impact on clinical outcomes (24,25). A post hoc analysis of a phase II trial of nab-P monotherapy in metastatic breast cancer also revealed no association of dose reduction with OS (26). Management of adverse events (AEs) by dose modification is suspected to increase the likelihood that a patient with pancreatic cancer can remain on treatment and consequently experience greater treatment exposure. However, there are few data to support this directly. Despite higher rates of dose reductions in the nab-P + Gem arm vs. the Gem arm in the MPACT trial, patients receiving nab-P + Gem had a longer treatment duration than those in the Gem-alone arm (median, 3.9 vs. 2.8 months). The goal of this exploratory analysis was to fully characterize the use of dose reduction or delay to manage toxicities and the effect of that dose modification on efficacy in the MPACT trial.
Methods
Patients and methods of the MPACT trial have been described previously, including a previous publication of a CONSORT diagram (18).
Patients
Adults (≥18 years of age) with histologically or cytologically confirmed metastatic adenocarcinoma of the pancreas and a KPS score of ≥70 were enrolled. Measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.0 was required (27). Additional eligibility criteria included adequate hepatic, hematologic, and renal function.
Study design and treatment
Patients were randomized 1:1 to receive a 30- to 40-minute intravenous infusion of nab-P 125 mg/m2, followed by an infusion of Gem 1,000 mg/m2 on days 1, 8, 15, 29, 36, and 43, or Gem alone 1,000 mg/m2 weekly for 7 of 8 weeks (cycle 1). Patients were treated on days 1, 8, and 15 every 28 days in subsequent cycles. Patient randomization was stratified by Karnofsky performance status, geographic region, and the presence of liver metastases. Treatment continued until either disease progression by RECIST or unacceptable toxicity.
Assessments
Tumor response was assessed every 8 weeks, and scans were evaluated by an independent radiology laboratory and by investigators using RECIST version 1.0.
Treatment-related AEs were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (28). Hematologic AEs were assessed by central laboratory testing. All patients were evaluated on days 1, 8, and 15 of each 28-day cycle for AEs that would require dose modification. Per protocol, doses could be reduced up to 2 times per therapeutic agent (to 100 or 75 mg/m2 for nab-P and to 800 or 600 mg/m2 for Gem; dose reductions may or may not have been concomitant) in the event of unacceptable toxicity (Table 1, Table S1). Dose re-escalation was not allowed between cycles. Toxicities that required a delay of either drug for >21 days resulted in permanent discontinuation.
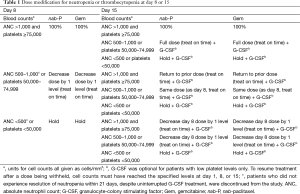
Full table

Full table
Investigators monitored treatment-related AEs and serious AEs, weekly central laboratory testing, and the rates of dose reductions, dose interruptions, and premature discontinuations of the study drug.
In all analyses, patients in the nab-P + Gem arm were designated as having had a dose reduction or delay based on modification of nab-P only. Doses not given within 2 days of the scheduled administration were considered dose delays. Reinitiation of treatment after a missed dose was required to be considered a delay.
Statistical analysis
All efficacy analyses were performed on the intent-to-treat (ITT) population, which was composed of all enrolled patients. The primary efficacy endpoint was OS, and the secondary endpoints were PFS and ORR. A multivariate analysis of OS was conducted that included the following covariates at baseline: age (<65 vs. ≥65 years), sex, KPS (70–80 vs. 90–100), geographic region (North America vs Eastern Europe, Western Europe, or Australia), pancreatic cancer primary location (head vs. other), presence of biliary stent, previous Whipple procedure, presence of liver metastases, presence of pulmonary metastases, peritoneal carcinomatosis, stage of diagnosis (IV vs. other), number of metastatic sites (1 vs. 2 vs. 3 vs. 4 vs. 5 vs. 6), and level of CA19-9 [< upper limit of normal (ULN) vs. ULN to 59× ULN vs. >59× ULN]. To assess the specific effect of each on OS, dose reduction (yes vs. no) and dose delay (yes vs. no) were added to the list of covariates, as was treatment group for the ITT analysis separately. Similar analyses were carried out within each treatment arm to account for the finding that more dose modifications were required in the nab-P + Gem arm vs. the Gem arm. Otherwise, the effect of each factor (treatment arm and dose modification) could have been canceled by the other since they were related.
All statistical tests were 2-sided and performed using SAS 9.1 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Dose modifications
All patients initiated treatment at the per-protocol starting doses of nab-P 125 mg/m2 + Gem 1,000 mg/m2 or Gem alone at 1,000 mg/m2. The median relative dose intensities were 81% for nab-P and 75% for Gem in the combination arm and 85% for Gem alone (18). The majority of patients with a nab-P dose reduction (104/172, 60%) required only 1 dose reduction; 33% had 2 dose reductions. Dose delays in the nab-P + Gem arm occurred in 71% of patients for nab-P and 70% for Gem (Table 2). The rate of dose delay in the Gem-alone arm was 57%. Most patients with a nab-P dose delay (163/300; 54%) had either 1 or 2 dose delays. Most nab-P dose reductions (155/257; 60%) and dose delays (679/946; 72%) occurred after the first 3 months (two cycles) of treatment.
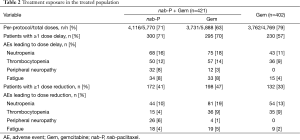
Full table
The main reasons for dose reductions and delays of nab-P were similar and included neutropenia, peripheral neuropathy, thrombocytopenia, and fatigue. The AEs that most often led to dose reductions or delays for Gem in each arm were neutropenia and thrombocytopenia (Table 2).
The AEs that led to dose reduction of nab-P by treatment cycle are represented in Figure 1A. Neutropenia was the AE that led to the highest rate of dose reduction throughout treatment. The percentages of dose reductions of nab-P due to neutropenia and thrombocytopenia were 25% and 10%, respectively, in cycle 1 and 27% and 10%, respectively, over cycles 1 to 5. The rates of dose reductions of nab-P due to peripheral neuropathy increased from 4% in cycle 1 to 14% over cycles 1 to 5. As expected, the percentages of patients in each treatment arm who received full doses of each drug decreased with increasing numbers of cycles (Figure 1B).
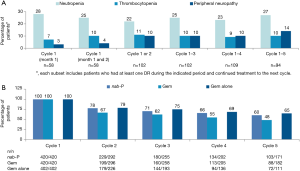
Treatment exposure based on dose modifications
To determine whether potential markers of frailty might associate with treatment exposure in this trial, the rates of dose reduction were examined in subsets of patients categorized by age and performance status. Neither of these factors appeared to influence the rate of dose reduction in either treatment arm (Table 3). In addition, the baseline characteristics for each treatment arm appeared similar between the ITT population and the patients who underwent dose reductions or delays (Table S2).

Full table
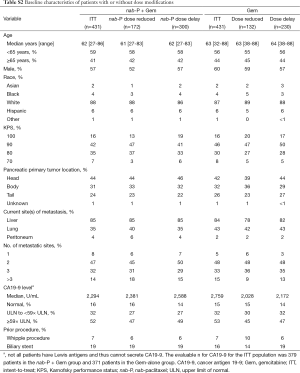
Full table
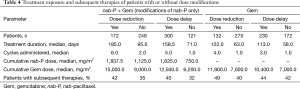
In the nab-P + Gem arm, patients who underwent dose reductions or delays of nab-P had a greater extent of treatment exposure than those who did not in terms of treatment duration, number of cycles administered, and cumulative dose of nab-P delivered (Table 4). Similar trends were observed for Gem in the Gem-alone arm. Rates of subsequent therapies appeared to be similar for patients who did or did not undergo a dose modification (Table 4).
Full table
Effect of dose modification on efficacy
Patients were divided into subgroups based on whether they underwent at least one dose reduction or not, and the efficacy outcomes for each of these subgroups were compared within each treatment arm. Efficacy appeared to be better for patients who underwent dose modification than for those who did not in each arm. In the nab-P + Gem arm, the ORRs in patients who did not (n=249) vs. did (n=172) undergo a dose reduction were 16% vs. 34%, respectively (RRR, 0.49; 95% CI, 0.34–0.69; P<0.0001). PFS was shorter for patients who did not undergo dose reductions vs. those who did (median, 3.8 vs. 8.8 months; HR, 2.62; 95% CI, 2.01–3.42; P<0.0001), as was OS (Figure 2A). Better efficacy was also observed for patients who underwent dose reductions in the Gem-alone arm. The ORRs for patients who did not (n=270) vs. did (n=132) undergo a dose reduction were 4% vs. 14%, respectively (RRR, 0.31; 95% CI, 0.16–0.62; P=0.0004). As in the nab-P + Gem arm, PFS in the Gem arm was shorter for patients who did not undergo a dose reduction vs. those who did (median, 3.5 vs. 5.5 months; HR, 2.13; 95% CI, 1.61–2.83; P<0.0001), as was OS (Figure 2B).
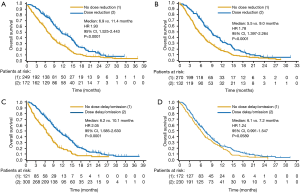
Similar efficacy trends favoring patients who underwent at least one dose delay were observed for patients who underwent at least one dose reduction. In the nab-P + Gem arm, patients who did not undergo a dose delay (n=121) had a lower ORR (10% vs. 29%; RRR, 0.34; 95% CI, 0.19–0.60; P<0.0001), a shorter PFS (median 3.4 vs. 6.6 months; HR, 2.80; 95% CI, 2.13–3.69; P<0.0001), and a shorter OS (Figure 2C) vs. those who did (n=300). In the Gem-alone arm, patients who did not undergo a dose delay (n=172) also had a lower ORR (3% vs. 11%; RRR, 0.32; 95% CI, 0.14–0.77; P=0.0061), a shorter PFS (median, 3.4 vs. 5.0 months; HR, 1.70; 95% CI, 1.31–2.21; P<0.0001), and a shorter OS (Figure 2D) vs. those who did (n=230), although the difference in OS did not reach statistical significance.
Additional analyses of dose modification and efficacy
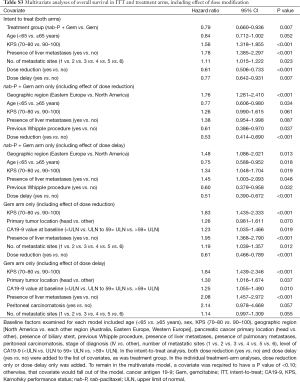
A stepwise multivariate analysis of OS was performed, which included a number of baseline factors as well as treatment group, dose reduction, and dose delay. Both dose reduction (HR, 0.61; P<0.001) and dose delay (HR, 0.77; P=0.007) were significantly associated with longer OS (Table S3). Treatment group, KPS, and presence of liver metastases were also significant predictors, as shown in a prior analysis (29), as was number of metastatic sites. Separate multivariate analyses were performed within each treatment arm to account for the statistical issue of higher rates of dose modifications in the nab-P + Gem arm (Table S3); dose reduction remained significantly associated with longer OS in each treatment arm, whereas dose delay was only significant in the nab-P + Gem arm (P=0.055 for the Gem arm).
Full table
OS was also assessed in an analysis that excluded patients who had disease progression before week 8 (cycle 1) to account for the possible influence of bias from the fact that early progression might have precluded dose modification (Table S4). Within each arm, patients with a dose reduction had a longer OS than patients who did not, and patients with a dose delay also had a longer OS than patients who did not.

Full table
To focus on patients with a presumed favorable biology, OS was assessed only in patients with a duration of tumor response above the median (median =6.03 months for the 130 patients who had a response). The Gem-only arm had only 11 patients with a duration of response above the median, which did not allow for statistical comparisons for dose reductions or delays. However, in the nab-P + Gem arm, which had 54 patients with a duration of response above the median, both dose reduction (HR, 0.24; P<0.001) and dose delay (HR, 0.04; P<0.001) were significantly associated with longer OS.
Discussion
The MPACT trial established nab-P 125 mg/m2 + Gem 1,000 mg/m2 as a new standard therapy for advanced pancreatic cancer (3,18), but very little published information is available on the efficacy of the regimen after dose modification. In the nab-P + Gem arm, at least 1 nab-P dose modification was associated with longer OS and most dose modifications occurred after the second cycle (i.e., 3 months of treatment). Together, this suggests that the regimen with the starting dose of nab-P 125 mg/m2 + Gem 1,000 mg/m2 was feasible and dose modifications to alleviate toxicities were not detrimental. Most patients (60%) with a nab-P dose reduction had only 1 dose reduction, and 54% of patients with a nab-P dose delay had 1 or 2 dose delays during treatment. The longer median duration of treatment in the nab-P + Gem vs. Gem arm (3.9 vs. 2.8 months) (18) likely reflects the improved efficacy of the combination and resulted in a higher proportion of patients in the nab-P + Gem arm requiring dose modification compared with the Gem-alone arm.
The AEs that led to the majority of dose modifications in the nab-P + Gem arm were neutropenia, thrombocytopenia, peripheral neuropathy, and fatigue. Although neutropenia and thrombocytopenia led to reductions of both drugs, peripheral neuropathy was responsible for nab-P reduction in 6% of patients and dose delay in 8% of patients in that arm but no dose reductions in the Gem-alone arm.
This analysis also evaluated associations between dose modifications and specific baseline characteristics and between dose modifications and efficacy. Older age and poorer performance status were not associated with a higher rate of dose modifications (Table 3). However, an interesting association was observed between dose modification and efficacy. In each treatment arm, patients with at least one dose reduction had a significantly higher ORR and longer OS and PFS than did patients without a dose reduction. Similar associations were observed for patients who did vs. did not undergo at least one dose delay, although in the Gem-alone arm the difference in OS for patients with vs without a dose delay was not statistically significant (P=0.0589).
The reasons for the apparent link between efficacy and dose modification are not immediately clear. One possible explanation is that some patients may be inherently more sensitive to the drugs than others, potentially leading to increased efficacy, more prolonged exposure, and increased chance of experiencing a toxicity requiring dose modification. An alternative possibility is that dose modification in response to a toxicity may have allowed patients to receive greater treatment exposure (more cycles and higher cumulative dose) and that this greater exposure resulted in better clinical outcomes. Although the available data do not allow a definitive explanation for the association of dose modification with efficacy, a number of analyses presented here minimize the likelihood that the link is an artifact attributable to the presumed lower rate of dose modification among patients with early disease progression or death.
Although we are not aware of any studies in pancreatic cancer that have demonstrated a link between dose modification and better efficacy, associations have been observed between efficacy and the development of rash in patients treated with erlotinib for pancreatic cancer or non-small cell lung cancer (30). In pancreatic cancer, the presence of grade ≥2 rash was significantly associated with longer OS (HR, 0.47; 95% CI, 0.34–0.64; P<0.001) and PFS (HR, 0.46; 95% CI, 0.33–0.65; P<0.001). Also, in the adjuvant treatment of stage III colon cancer, an association was reported between a capecitabine-specific adverse reaction and long-term efficacy (31). A post hoc analysis of data from the X-ACT trial demonstrated that capecitabine-treated patients who developed hand-foot syndrome had higher 5-year disease-free and OS rates compared with patients who did not develop the syndrome (61.3% vs. 55.5% and 73.8% vs. 66.3%, respectively).
The MPACT trial has firmly established nab-P 125 mg/m2 + Gem 1,000 mg/m2 the first 3 of 4 weeks as a novel, effective, and fairly well tolerated treatment option in patients with metastatic pancreatic cancer. Based on the present data investigating the use of dose reduction and/or delay of chemotherapeutic drugs to manage toxicities, as well as the effect of dose modification on efficacy, the following conclusions can be drawn: (I) there is a need to carefully monitor patients for neutropenia, thrombocytopenia, peripheral neuropathy, and fatigue; (II) the methods of dose modification outlined in the trial protocol were effective and should be followed in routine clinical practice; (III) these dose adjustments can be carried out with the knowledge that they do not reduce the efficacy of the established 125 mg/m2 starting nab-P dose. Our data highlight the importance of titrating the dose of chemotherapeutic drugs to the individual patient, a strategy that, according to this exploratory analysis, seems to result in an optimal therapeutic outcome.
Acknowledgements
Funding provided by Celgene Corporation. Writing assistance was provided by John R. McGuire, PhD, Meditech Media, LLC. Biostatistical support was provided by Peng Wu, PhD, MS, Celgene Corporation.
Footnote
Conflicts of Interest: WS—reports grants, personal fees (for consultant, advisory board & invited speaker activities) and non-financial support (study medication drug supply) from Celgene Corporation, during the conduct of the study; RKR—reports grants and personal fees from Celgene Corporation, outside the submitted work; MM—reports grants from Celgene Corporation, during the conduct of the study; personal fees from Celgene Corporation outside the submitted work; TG—nothing to disclose; DG—reports grants from Celgene Corporation, during the conduct of the study; grants from Pfizer, grants from Amgen, outside the submitted work; PH—reports consulting for Celgene Corporation during the conduct of the study; VK—reports honoraria for consulting and lectures by Celgene Corporation; HL—reports employment, stock ownership from Celgene Corporation; DM—reports employment, stock ownership from Celgene Corporation; AR—reports employment, stock ownership from Celgene Corporation; DDVH—reports consultant, honoraria, and research funding, Celgene Corporation.
Disclaimer: The authors were fully responsible for all content and editorial decisions for this manuscript.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- National Comprehensive Cancer Network, editor. Clinical practice guidelines in oncology: pancreatic adenocarcinoma. V 2. 2014.
- Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002;20:3270-5. [Crossref] [PubMed]
- Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004;22:3776-83. [Crossref] [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [Crossref] [PubMed]
- Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 2005;16:1639-45. [Crossref] [PubMed]
- Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol 2006;24:4441-7. [Crossref] [PubMed]
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24:3946-52. [Crossref] [PubMed]
- Stathopoulos GP, Syrigos K, Aravantinos G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer 2006;95:587-92. [Crossref] [PubMed]
- Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007;25:2212-7. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-8. [Crossref] [PubMed]
- Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009;27:3778-85. [Crossref] [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [Crossref] [PubMed]
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605-10. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- James ES, Cong X, Yao X, et al. Final analysis of a phase II study of Yale-modified FOLFIRINOX (mFOLFIRINOX) in metastatic pancreatic cancer (MPC). J Clin Oncol 2015;33:abstr 395.
- Maroun J, Ko YJ, Ghafoor A, et al. A registry of real-world clinical practice on the use of FOLFIRINOX (FFX) in advanced pancreatic cancer (aPC) patients in Canada. Proceedings of the European Cancer Congress 2015:abstr 2340.
- Goldstein D, El-Maraghi RH, Hammel P, et al. Analysis of updated overall survival and prognostic effect of neutrophil-to-lymphocyte ratio and CA 19-9 from the phase III MPACT study of nab-paclitaxel plus gemcitabine versus gemcitabine for patients with metastatic pancreatic cancer. J Clin Oncol 2014;32:abstr 4027.
- Arriagada R, Le Chevalier T, Pignon JP, et al. Initial chemotherapeutic doses and survival in patients with limited small-cell lung cancer. N Engl J Med 1993;329:1848-52. [Crossref] [PubMed]
- Samson MK, Rivkin SE, Jones SE, et al. Dose-response and dose-survival advantage for high versus low-dose cisplatin combined with vinblastine and bleomycin in disseminated testicular cancer. A Southwest Oncology Group study. Cancer 1984;53:1029-35. [Crossref] [PubMed]
- Nagel CI, Backes FJ, Hade EM, et al. Effect of chemotherapy delays and dose reductions on progression free and overall survival in the treatment of epithelial ovarian cancer. Gynecol Oncol 2012;124:221-4. [Crossref] [PubMed]
- Brunetto AT, Carden CP, Myerson J, et al. Modest reductions in dose intensity and drug-induced neutropenia have no major impact on survival of patients with non-small cell lung cancer treated with platinum-doublet chemotherapy. J Thorac Oncol 2010;5:1397-403. [Crossref] [PubMed]
- Gradishar WJ, Krasnojon D, Cheporov S, et al. A randomized phase II trial of first-line metastatic breast cancer (MBC) patients: Sub-set analysis of albumin-bound paclitaxel (ab-pac) given weekly at 150 mg/m2. San Antonio Breast Cancer Symposium 2011:poster P5-19-13.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research And Treatment of Cancer, National Cancer Institute of the United States, National Cancer institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3notice.pdf. Updated August 9, 2006. Accessed 04/08, 2015.
- Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015;20:143-50. [Crossref] [PubMed]
- Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 2007;13:3913-21. [Crossref] [PubMed]
- Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190-7. [Crossref] [PubMed]


