Immunohistochemistry and scoring of Ki-67 proliferative index and p53 expression in gastric B cell lymphoma from Northern African population: a pilot study
Introduction
In recent years, many studies stress the prognostic significance of the evaluation of tumor biology and, linked to it, processes of cell proliferation. The proliferative activity of the tumor is related to its growth rate, providing a recognized prognostic index, related to survival of patients with various types of tumors (1,2). Several methods to assess proliferative fraction of tumor cells have been using e.g., flow cytometry, autoradiography and evaluation of nucleolar organizer region. At present, immunohistochemical (IHC) methods represent an effective approach, detecting antigens typical for cells in the cell cycle (e.g., Ki-67, PCNA, Mcm-2) (3,4). Ki-67 antigen represents a non-histone nuclear antigen present only in proliferating cells. Its role as a proliferative index (PI) marker is due to the fact that, Ki-67 is expressed during the active phases of cell cycle, which are G1, S, G2, and mitosis. Its strict association with cell proliferation and its co-expression with other well-known markers of proliferation indicate a pivotal role in cell division. Therefore it is an excellent marker for determining the growth fraction of a given cell population (5). Experimental and clinical data indicate that tumor progression and malignancy are associated with increased angiogenesis and higher Ki-67 PI (6). Hall et al. (7) and Hussain and Harris (8), made clinical classification of non-Hodgkin’s lymphomas into high- and low-grade lymphomas which was shown to be mirrored by differences in Ki-67 staining. One of the major pathways in controlling cell cycle is p53 tumor suppressor, the p53 gene may block the progression of cell growth cycle and trigger apoptosis in response to DNA damage (9). p53 abnormalities such as gene mutation and depletion can lead to the altered intracellular signal transduction pathways as well as loss of the regulation of cell growth, apoptosis, and DNA repair, which are responsible for carcinogenesis (10). The biological activities of p53 are attributed to its ability to arrest the cell cycle at G1 or G2 phase, to induce apoptosis and to maintain genomic stability by modulating DNA repair, replication and recombination (11). Many observations suggest the involvement of p53 in lymphoma-genesis including: (I) p53 gene mutations are found in about 25–30% of NHL (12); (II) p53 immunoreactivity is a frequent finding in NHL (13).
This study was conducted to evaluate Ki-67 (PI) level and p53 expression as an IHC markers in gastric B cell lymphoma and asses the correlation between those two markers and different clinico-pathological data (age, sex, tumor location and histological type).
Methods
Patients and tissue samples
Twenty paraffin blocks from patients with gastric B cell lymphoma were collected from the Department of Pathology, Central University Hospital of Sidi Bel Abbes City (Western Algeria) for the period 2007–2013, including 13 blocks of MALT B-cell lymphoma and 7 blocks of DLBCL. These specimens were paraffin embedded in the same center. The cases were stained with haematoxyline and eosin (H&E) for routine histological examination. An absolute confidentiality of the patients’ vital information was maintained for ethical purposes and an ethical approval was obtained from institutions in which the study was carried out.
Immuno-histochemical staining
For immuno-histochemical study, the streptavidin-biotin-peroxidase method was performed on paraffin sections. The following monoclonal antibodies were used: anti-Ki-67 (clone SP6, Diagomics, dilution 1/400°, incubation 32 min, pretreatment CC1 36 min); anti-p53 (clone DO-7, DAKO, dilution 1/50°, incubation 32 min, pretreatment CC1 64 min) . The phenotype of the lesions was evaluated using anti-CD20 and anti-CD3 anti-bodies. The 4-µm sections were deparaffinized with xylene twice during 10 min for each, rehydrated in a gradient series of alcohol (100%, 95% and 45% alcohol), and rinsed by PBS. Each section was covered with 0.3% peroxy acetic acid for 15 min to block endogenous peroxidase activity, microwaved for antigen retrieval (100 W, 5 min × 3 min), and cooled in the room temperature for 20 min. These sections were first incubated with primary antibody at room temperature, and then rinsed once with PBS. This was followed by incubation with a secondary antibody streptavidin-biotin-peroxidase complex for 30 min in room temperature at 37° as well as another rinsed once with PBS. Slides were then treated with streptavidin peroxidase reagent for 10 min and rinsed with PBS twice. The sections were visualized with 3,3'-diaminobenzidine (DAB) for 5 min, counter stained with hematoxylin for 5 min, washed gently under running water ammoniac and differentiated by and mounted in mounting medium for observation under microscope.
For the tissue evaluation of Ki-67, each slide was scored based on the percentage of positively stained malignant nuclei. The following ranges were used: 0% to 20%, >20% to 40%, >40% to 60%, >60% to 80%, and >80% to 100%. According to the recommended classification in previous studies (14,15), samples with Ki-67 nuclear staining equal or above 40% were considered having a high PI, whereas nuclear positivity below 40% was considered a low PI. p53 was considered overexpressed when ≥30% of the malignant nuclei were positive.
Statistical analysis
Statistical analysis was performed using the SPSS 20.0. Ki-67, p53 expression as well as their correlations with clinico-pathological parameters were performed by chi2 test. Significant differences were accepted at P<0.05.
Results
Clinico-pathologic features
From 2007 to 2013, 20 patients with gastric B cell lymphoma were identified and included in our study. The mean age of the patients was 60, 65 years, with a range of 35–78 years. Most of patients included in this study were 15 (75%) cases over 50 years. Gender distribution of cases, showed male predominance 16 (80%) compared with female 4 (20%), and a male to female ratio of about 4:1.
Regarding the site of gastric lymphoma, most of the patients had tumors located mainly in the antrum of the stomach (12 patients), location in the body was seen in 2 patients, and it was fundus in 6 patients.
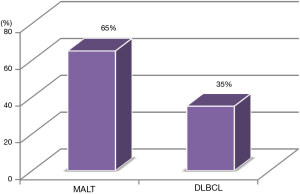
The study sample comprised 13 (65%) patients who were classified as MALT lymphomas and 7 (35%) as diffuse large B-cell lymphomas (Figure 1). Macroscopically, 9 of 13 MALT and 4 of 7 DLBCL were classified as ulcer type, 4 of the MALT, 2 of DLBCL as infiltrate and the one case of DLBCL was classified as polyp (Table 1).
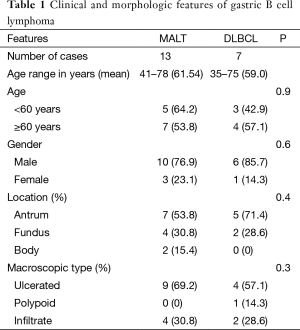
Full table
Ki-67 distribution in gastric B cell lymphoma
The percentage of Ki-67-positive cells in the tissues from 20 patients ranged from 2% to 90%, with a mean of 30.20%. Males presented a greater percentage of stained cells than females (mean Ki-67 PI: 32.81% versus 28.80%). The association between mean Ki-67 PI and histological type showed that the mean Ki-67 was 5.69% in low grade (MALT) and 75.71% in high grade (DLBCL). Significant difference was observed between Ki-67 PI and histological grade advanced (P=0.001). However, no significant statistical correlation was found between Ki-67 PI and clinic-pathological parameters like age (P=0.3), sex (P=0.3), macroscopic features (P=0.4), location of tumor (P=0.2) (Table 2, Figure 2).
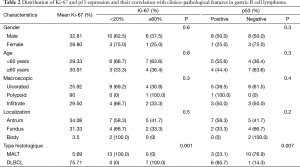
Full table
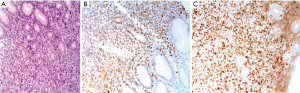
p53 expression in gastric B cell lymphoma
The expression of p53 protein was observed in nine samples (45%). The frequency of p53 positive specimens in low grade (MALT) (33.3%) appeared to be lower than in high grade (DLBCL) (66.7%); the frequency of positivity was significantly increased as the histological grade advanced (P=0.007). However, no significant correlation was found between the p53 expression and clinico-pathological parameters: age, sex, macroscopic feature or disease location (Table 2, Figure 2). We found a statistically significant correlation between p53 and Ki-67 (P=0.007). p53-negative patients presented lower Ki-67 values than p53-positive patients (Table 3).

Full table
Discussion
Primary gastric lymphomas are rare. Although their incidence rate is increasing, they comprise less than 10% of gastric malignancies. Despite this rarity, stomach accounts for more than two-third of all extra-nodal non-Hodgkin lymphomas (16).
The mean age at diagnosis of patients with gastric B cell lymphoma is 60, 65 years, which is comparable to the results published in the literature. In fact, this number is very close to the mean age reported in Germany and Japan, which was 60 and 60, 5 years respectively (17,18). However, it was lower in other Asian countries; it doesn’t exceed the 50 years in china (19) and 42 years in Iraq (20). The M/F sex ratio in our studied population is higher than what has been reported from china (1.18) (21) and France (1.24) (22), but it is contrast to the study by Ansari et al., which showed a female predominance (23).
In general, GL more frequently involves antrum, corpus, and cardia (24). In our series of 20 patients, most of the patients (60%), antrum was the main involved site, accounting for 60% cases. The histology types in this study had shown similar patterns of frequency compared to report of Al-Shehabi et al. (25) and Xie et al. (16), in which MALT was the dominant histology types, while reports from Jordan (26) and Iran (23) have shown a predominance of DLBCL.
Ki-67 is a nonhistone protein first identified in 1991 by Gerdes et al. (27). Because it is expression in all phase of the cell cycle except the resting stage (G0), it has been used as a proliferation marker in numerous cancers including lymphoma (28). Several researchers tried to establish usefulness of Ki-67 index in distinguishing indolent and aggressive lymphomas. Broyde et al. evaluated Ki-67 index in 319 newly diagnosed cases of NHL. There was a statistically significant increase in mean Ki-67 index from 26.6% for indolent lymphomas to 67.2% for aggressive lymphomas to 97.6% for very aggressive lymphomas. They established a Ki-67 index of 45% to differentiate indolent from aggressive lymphomas (29) and increased Ki-67 index has been shown to correlate with increased grade of lymphoma (30). This study demonstrates the differences in the range and mean percentage of Ki-67 in low and high grade lymphomas. Our results were consistent with these previous observations, since all of our tested gastric DLBCL had high Ki-67 index ≥80%, while the majority (85%) of the gastric MALT had Ki-67 ≤20%. Statistical analysis on our lymphomas also showed higher Ki-67 levels were significantly associated with high grade DLBCL lymphoma (P=0.001). Petit et al. assessed proliferation index in 90 indolent lymphoplasmacytic and marginal zone lymphoma. They found mean Ki-67 index of 20% being associated with low overall survival (31). On the basis of the Kiel classification and the PI assessment by proliferating cell nuclear antigen staining in histologic sections, the mean PI reported in low-grade lymphomas was 39.5 and 75.7 in high-grade lymphomas (32). Correlations using the working formulation classification described mean MIB-1 PI of 29.7% for low-grade lymphomas, 53.1% for intermediate-grade, and 75.1% for high-grade lymphomas (33). In accordance with results reported by Szczurazek et al. (34), no significant relationship was shown between Ki-67 and clinico-pathological features of the patients (age, sex, location, macroscopic features).
As we know that p53 is a transcription factor that induces the expression of genes involved in cell cycle arrest or apoptosis in response depending on the biologic context (35). The finding of p53 protein overexpression in NHL is in agreement with the previous studies and suggests a possible role for p53 in lymphomagenesis (12-36). p53 over-expression has been shown to correlate with mutations in TP53 (37). This may relate to decreased rate of degradation of the mutant protein, leading to elevated cytoplasmic accumulation. p53 immunohistochemistry may be useful in evaluating primary GI lymphomas, because p53 accumulation has been shown to be more common in high grade lymphomas than low grade lymphomas (30). Moller et al. showed that p53 protein overexpression, defined as ≥20% of tumor cells positive for p53, was 80-90% sensitive and 100% specific in predicting TP53 mutations in DLBCLs (38). In our series, high grade gastric DLBCL were more significantly associated with p53 overexpression than low grade gastric MALT (P=0.007). Thus our data corroborates the prior studies which have suggested mutations in TP53 may relate to progression from low grade to high grade lymphoma, as well as increased risk of relapse (38,39).
Conclusions
Our preliminary results showed that high Ki-67 PI distribution and p53 overexpression in gastric B cell lymphoma were associated with DLBCL type and p53 expression also correlated with a greater PI. However, in this selected group of patients, no correlation was noted between these markers expression and clinico-pathological features.
Acknowledgements
The authors would like to thank the members of the Pathology Services of the University Hospital Center of Sidi Bel Abbes for their invaluable support, guidance, and educational insight.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gerdes J. Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin Cancer Biol 1990;1:199-206. [PubMed]
- Pastuszewski W, Dziegiel P, Krecicki T, et al. Prognostic significance of metallothionein, p53 protein and Ki-67 antigen expression in laryngeal cancer. Anticancer Res 2007;27:335-42. [PubMed]
- Gryczynski M, Pastuszewska W. Selected aspects of apoptosis and cell proliferation in laryngeal carcinoma. Otolaryngologia 2002;1:151-60.
- Szelachowska J, Dziegiel P, Jelen-Krzeszewska J, et al. Mcm-2 protein expression predicts prognostic better than Ki-67 antigen in oral squamocellular carcinoma. Anticancer Res 2006;26:2473-8. [PubMed]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311-22. [Crossref] [PubMed]
- Patruno R, Zizzo N, Zito AF, et al. Microvascular density and endothelial area correlate with Ki-67 proliferative rate in the canine non-Hodgkin's lymphoma spontaneous model. Leuk Lymphoma 2006;47:1138-43. [Crossref] [PubMed]
- Hall PA, Richards MA, Gregory WM, et al. The prognostic value of Ki67 immunostaining in non-Hodgkin's lymphoma. J Pathol 1988;154:223-35. [Crossref] [PubMed]
- Hussain SP, Harris CC. p53 biological network: at the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J Nippon Med Sch 2006;73:54-64. [Crossref] [PubMed]
- Prokocimer M, Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood 1994;84:2391-411. [PubMed]
- Xerri L, Bouabdallah R, Camerlo J, et al. Expression of the p53 gene in Hodgkin's disease: dissociation between immunohistochemistry and clinicopathological data. Hum Pathol 1994;25:449-54. [Crossref] [PubMed]
- Blaszyk H, Hartmann A, Cunningham JM, et al. A prospective trial of midwest breast cancer patients: a p53 gene mutation is the most important predictor of adverse outcome. Int J Cancer 2000;89:32-8. [Crossref] [PubMed]
- Klumb CE, Furtado DR, de Resende LM, et al. DNA sequence profile of TP53 gene mutations in childhood B-cell non-Hodgkin's lymphomas: prognostic implications. Eur J Haematol 2003;71:81-90. [Crossref] [PubMed]
- Echezarreta G, Marcos B, Sanz C, et al. Value of protein 53 expression in lymphoma. Its correlation with genetic mutations. Sangre (Barc) 1997;42:369-75. [PubMed]
- Allegra CJ, Paik S, Colangelo LH, et al. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes' B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol 2003;21:241-50. [Crossref] [PubMed]
- van Triest B, Pinedo HM, Blaauwgeers JL, et al. Prognostic role of thymidylate synthase, thymidine phosphorylase/platelet-derived endothelial cell growth factor, and proliferation markers in colorectal cancer. Clin Cancer Res 2000;6:1063-72. [PubMed]
- Xie L, Shen LD, Qing C, et al. Correlational study of vascular endothelial growth factor expression and microvessel density in primary malignant gastric lymphoma. Med Oncol 2012;29:1711-5. [Crossref] [PubMed]
- Koch P, Probst A, Berdel WE, et al. Treatment results in localized primary gastric lymphoma: Data of patients registered within the German multicenter study (GIT NHL 02/96). J Clin Oncol 2005;23:7050-9. [Crossref] [PubMed]
- Takahashi I, Maehara Y, Koga T, et al. Role of surgery in the patients with stage I and II primary gastric lymphoma. Hepatogastroenterol 2003;50:877-82. [PubMed]
- Luo ZG, Feng FY, Zhang P, et al. Clinical analysis of 68 patients with primary gastric lymphoma. Ai Zheng 2004;23:1692-5. [PubMed]
- Al-Bahrani Z, Al-Mondhiry H, Bakir F, et al. Primary gastric lymphoma. Review of 32 cases from Iraq. Ann R Coll Surg Engl 1982;64:234-7. [PubMed]
- Huang J, Jiang W, Xu R, et al. Primary gastric non-Hodgkin’s lymphoma in Chinese patients: clinical characteristics and prognostic factors. BMC Cancer 2010;10:358. [Crossref] [PubMed]
- Danzon A, Belot M, Maynadié M, et al. Incidence and survival of gastric non-Hodgkin’s lymphoma: a population-based study from the Association of the French Cancer Registries (FRANCIM). Acta Oncol 2009;48:977-83. [Crossref] [PubMed]
- Ansari M, Nasrolahi H, Abbas Kani A, et al. Primary Non-Hodgkin’s Lymphoma of Stomach: To Report 54 Patients and Analysis of Major Reported Series. Lymphoma 2013;2013:583826-34.
- Ferrucci PF, Zucca E. Primary gastric lymphoma pathogenesis and treatment:what has changed over the past 10 years? Br J Haematol 2007;136:521-38. [Crossref] [PubMed]
- Al-Shehabi ZA, Zezafon HB, Saleh RS. Clinicopathological study of primary gastric lymphoma. Saudi Med J 2007;28:1506-10. [PubMed]
- Bani-Hani KE, Yaghan RJ, Matalka II. Primary gastric lymphoma in Jordan with special emphasis on descriptive epidemiology. Leuk Lymphoma 2005;46:1337-43. [Crossref] [PubMed]
- Gerdes J, Li L, Schlueter C, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 1991;138:867-73. [PubMed]
- Dziegiel P, Salwa-Zurawska W, Zurawski J, et al. Prognostic significance of augmented metallothionein (MT) expression correlated with Ki-67 antigen expression in selected soft tissue sarcomas. Histol Histopathol 2005;20:83-9. [PubMed]
- Broyde A, Boycov O, Strenov Y, et al. Role and prognostic significance of the Ki-67 index in non-Hodgkin’s lymphoma. Am J Hematol 2009;84:338-43. [Crossref] [PubMed]
- Li HL, Sun BZ, Ma FC. Expression of COX-2, iNOS, p53 and Ki-67 in gastric mucosa- associated lymphoid tissue lymphoma. World J Gastroenterol 2004;10:1862-6. [Crossref] [PubMed]
- Petit B, Chaury MP, Le Clorennec C, et al. Indolent lymphoplasmacytic and marginal zone B-cell lymphomas:absence of both IRF4 and Ki67 expressionidentifies a better prognosis subgroup. Haematologica 2005;90:200-6. [PubMed]
- Rabenhorst SH, Burini RC, Schmitt FC. Proliferating cell nuclear antigen (PCNA) in non-Hodgkin’s lymphomas correlation with working formulation and Kiel classification in formalin-fixed paraffin-embedded material. Pathology 1996;28:12-6. [Crossref] [PubMed]
- Kalogeraki A, Tzardi M, Panagiotides I, et al. MIB1 (Ki-67) expression in non-Hodgkin’s lymphomas. Anticancer Res 1997;17:487-91. [PubMed]
- Szczuraszek K, Mazur G, Jelen M, et al. Prognostic Significance of Ki-67 Antigen Expression in Non-Hodgkin's Lymphomas. Anticancer Res 2008;28:1113-8. [PubMed]
- Sánchez-Beato M, Sánchez-Aguilera A, Piris MA, et al. Cell cycle deregulation in B-cell lymphomas. Blood 2003;101:1220-35. [Crossref] [PubMed]
- Burra U, Shanthi P, Krishnan KB, et al. P53 and PCNA in Non-Hodgkin’s lymphoma— an immunohistochemical evaluation. Indian J Pathol Microbiol 2000;43:61-4. [PubMed]
- Hall PA, Lane DP. p53 in tumour pathology: can we trust immunohistochemistry?- Revisited! J Pathol 1994;172:1-4. [Crossref] [PubMed]
- Moller MB, Gerdes AM, Skjodt K, et al. Disrupted p53 function as predictor of treatment failure and poor prognosis in B- and T-cell non-Hodgkin's lymphoma. Clin Cancer Res 1999;5:1085-91. [PubMed]
- Dierlamm J, Stefanova M, Wlodarska I, et al. Analysis of the P53, RB/D13S25, and P16 tumor suppressor genes in marginal zone B-cell lymphoma: An interphase fluorescence in situ hybridization study. Cancer Genet Cytogenet 2000;120:1-5. [Crossref] [PubMed]