A patient who showed a pathologically complete response after undergoing treatment with XELOX plus bevacizumab for synchronous liver metastasis of grade H2 from sigmoid colon cancer
Introduction
Surgical resection is the only curative option for liver metastases from colorectal cancer (CRLM); however, the incidence of unresectable CRLM remains high (1). Recently, systemic chemotherapy for unresectable metastatic colorectal cancer has remarkably progressed. In a retrospective analysis, more than 12% of cases of initially unresectable CRLM converted to resectable status after the patients showed responses to chemotherapy and were reported to show a good 5-year survival rate of more than 30% (2). Surgical procedures such as two-staged hepatectomy have also been developed for such cases (3,4). On the other hand, neoadjuvant chemotherapy followed by hepatic resection for initially resectable CRLM has also been suggested to be effective (5). Histopathological tumor regression due to preoperative chemotherapy has recently been recognized to be an important indicator of a significantly better prognosis in patients with CRLM (6). In addition, a pathological complete response to preoperative chemotherapy was associated with a 5-year survival of 75% in patients with CRLM compared to 33% in patients with a minor pathological response (7). However, Adam et al. (8) showed that the incidence of pathological complete responses (pCRs) is insufficient, at approximately 4%, and 71% of the CRLM measured less than 3.0 cm in diameter in cases of pCR. Smaller liver deposits may be associated with a higher incidence of pCR due to preoperative chemotherapy.
We herein report the case of a patient with synchronous solitary liver metastasis from sigmoid colon cancer. The maximum diameter of the liver deposit was 5.7 cm and its grade was therefore H2 according to the Japanese classification (9). A pCR was detected after the patient underwent deferred hepatic resection after neoadjuvant chemotherapy consisting of capecitabine, oxaliplatin and bevacizumab (XELOX + Bev). A pCR in a patient with H2 liver metastasis is considered rare and suggests that XELOX + Bev is efficacious as neoadjuvant chemotherapy.
Case presentation
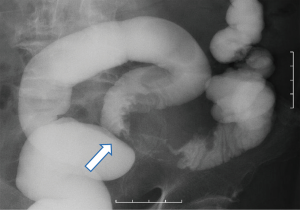
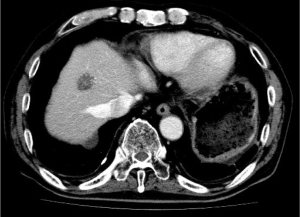
The patient was a 60-year-old male without any chief complaint. His previous history included atrial fibrillation, hypertension, hyperlipidemia and hyperuricemia. His family history was unremarkable. He underwent abdominal ultrasonography as part of a medical examination. A hepatic mass was detected and the patient was referred to our hospital. On a serum biochemical examination, the carcinoembryonic antigen (CEA) level was elevated to 67.8 ng/mL and the tumor-associated carbohydrate antigen19-9 (CA 19-9) level was elevated to 3,551.8 U/mL. Abdominal contrast-enhanced computed tomography showed a large and solitary low density mass of 5.7 cm in maximum diameter in segment 8 of the liver (Figure 1). No other distant metastases were detected. Colorectal endoscopy and a bowel barium enema revealed sigmoid colon cancer (Figure 2). According to these examinations, the final diagnosis was synchronous solitary liver metastasis from sigmoid colon cancer and the clinical progression of the disease was stage IV. The liver metastasis was classified as H2 according to the Japanese classification (9).

Although performing simultaneous resection with colectomy and hepatectomy was considered possible, treatment with deferred hepatectomy after sigmoidectomy followed by neoadjuvant chemotherapy was selected due to the risk of an early recurrence of the liver metastasis.
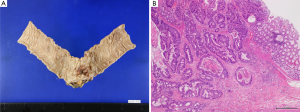
The patient then underwent curative laparoscopic sigmoidectomy. The histopathological findings were as follows: type-2 advanced sigmoid colon cancer, 35 mm × 25 mm in size, moderately differentiated adenocarcinoma, subserosal invasion and no lymph node metastasis (Figure 3A,B).
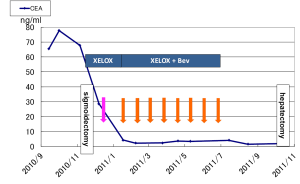
Treatment with neoadjuvant chemotherapy was started four weeks after the sigmoidectomy was performed. The patient received XELOX (1,000 mg/m2 of capecitabine and 130 mg/m2 of oxaliplatin) without Bev as the first cycle followed by XELOX + Bev (7.5 mg/kg). Our clinical trial (No. 2010-1814) to evaluate preoperative chemotherapy including XELOX + Bev in patients with initially resectable CRLM has been approved by the local ethics committee of St. Marianna University. Although nausea and diarrhea (both grade 2) were observed after the second cycle, the chemotherapy was continued as scheduled with almost no dose reductions or discontinuation. Initially, we planned to perform hepatic resection after six cycles of chemotherapy; however, the patient desired to continue chemotherapy because the tumor diameter had decreased to 2.5 cm on CT by the end of the sixth cycle and the CEA and CA19-9 levels had normalized to 9.3 U/mL and 3.5 ng/mL, respectively.
After the ninth cycle of XELOX + Bev, the patient developed an adhesive intestinal obstruction and was hospitalized. The size of the liver metastasis had decreased to 2.2 cm (Figure 4) and the CEA and CA19-9 levels were 1.9 ng/mL and 5.7 U/mL, respectively. After the intestinal obstruction was treated with conservative treatment, the patient finally consented to undergo liver resection. Twelve weeks after the ninth cycle of chemotherapy, the patient underwent curative metastasectomy of segment 8 instead of anterior sectionectomy of segments 5 and 8, which might have been needed before the chemotherapy was administered. A small intestinal adhesiotomy was added to the hepatectomy.
The resected liver specimen showed the tumor size to be 25 mm × 22 mm × 25 mm. There was an ash-white tone to the tumor tissue in section; however, the surgical margin was cancer negative (R0).
Histopathologically, the liver damage associated with the chemotherapy was mild and sinusoidal obstruction syndrome (SOS) of grade 1 was observed. Remarkable calcifications without cancer cells were detected in the tumor. The tumor consisted of complete necrotic tissue, and the chemotherapeutic response was pCR (Figure 5).

Postoperatively, the patient presented with an intestinal occlusion that was conservatively treated. The patient did not present with any liver-related complications. The patient’s progress is shown in Figure 6.
Nine months after the hepatectomy, the CEA and CA19-9 levels remained normalized and no recurrence was observed.
Discussion
Systemic chemotherapy for unresectable CRLM has remarkably progressed, and approximately 12% of cases are converted to resectable status during treatment with chemotherapy (2). It has also been reported that FOLFOX4 administered before and after hepatectomy for initially resectable CRLM can improve disease-free survival (5). However, the incidence of pCR following chemotherapy remains insufficient (6-8). In addition, a pCR in patients with liver metastasis is considered rare in cases of H2 or H3 disease classified according to the Japanese classification (9). We herein report the case of a patient who showed a pCR after undergoing neoadjuvant chemotherapy with XELOX + Bev for grade H2 synchronous solitary liver metastasis measuring 5.7 cm in diameter.
Adam et al. (8) reported 29 patients who showed pCRs. The average diameter of their liver deposits before chemotherapy was 2.9 cm, and 71% of the deposits that measured less than 3.0 cm in diameter. However, in that report, the rate of pCR in patients with deposits larger than 5 cm [H2 or H3 according to the Japanese classification (9)] was not stated.
Interestingly, Adam et al. reported that the radiological disappearance of liver deposits (rCR) is not consistent with a pCR (8). Benoist et al. performed extensive hepatectomies in patients with non-detectable liver deposits (rCR) in order to perform R0 resection (10). Histopathologically, only 20% of lesions with rCR are detected to show a pCR. Based on these findings, confirming a pCR before performing liver surgery remains difficult. Four factors have been reported to be independent predictive factors of pCRs: age 60 years or younger, metastases measuring 3 cm or smaller at diagnosis, a CEA level of 30 ng/mL or less at diagnosis and the occurrence of an objective response following chemotherapy (8). The present patient exhibited only an objective response following chemotherapy and did not fulfill the other three criteria. This finding may indicate why this case is considered rare.
Concerning the chemotherapeutic regimens leading to a pCR, Adam et al. reported that the majority of patients (66%) who show a pCR received FOLFOX, 7% received cetuximab and none had received bevacizumab (8). Rubbia-Brandt et al. showed that pCRs are obtained only in patients who receive FOLFOX or FOLFOXIRI and not in patients who receive treatment combined with cetuximab or bevacizumab (6). Inoue et al. reported the case of a patient with four liver metastases measuring 2.0 cm or smaller in diameter who showed a pCR after receiving modified FOLFOX6 + Bev (11). There are few reports of pCRs occurring after treatment with XELOX or XELOX + Bev. Klinger et al. reported that three of 50 patients (6%) receiving XELOX or FOLFOX showed a pCR, while 10 of the other 50 patients (20%) receiving XELOX + Bev showed a pCR (12). It was unclear whether patients with H2 liver metastases showed pCRs in their report. However, as shown in prospective studies, the administration of XELOX + Bev before hepatectomy can be effective for both initially unresectable (13) and resectable CRLM (14), and XELOX + Bev as neoadjuvant chemotherapy administered before hepatectomy seems to increase the rate of pCR.
Regarding the optimal duration of chemotherapy, Adam et al. (8) reported that the median number of chemotherapeutic cycles in patients who show a pCR is eight and that 62% of pCRs occur after the administration of first line chemotherapy. Klinger et al. showed that 20% of pCRs occur after six cycles of XELOX + Bev based on the evaluation of pathological responses among resected patients in a prospective study of six cycles of FOLFOX or XELOX +/- Bev (13,15). The patient in our case report showed a pCR after undergoing nine cycles of XELOX + Bev as the first line treatment.
Chemotherapy with oxaliplatin administered before hepatectomy may cause hepatic sinusoidal obstruction syndrome (SOS) or severe postoperative complications such as liver failure, especially in cases that involve major hepatectomy (16). On the other hand, bevacizumab has been reported to protect against SOS (17). In the present case, mild SOS of grade 1 was observed and no postoperative liver complications occurred. Postoperative liver failure has been reported to be correlated with the administration of more than nine cycles of chemotherapy before surgery (18). In the present case, it was necessary to obtain informed consent after the planned first six cycles of chemotherapy.
A pCR occurring after the administration of neoadjuvant chemotherapy is considered to predict a better prognosis after resection of CRLM. Blazer et al. (7) reported that the 5-year survival rate was 75% in 25 patients who showed a pCR, and Adam et al. reported a rate of 76% in such patients (8). As the sigmoid colon cancer was curatively resected and no extrahepatic disease was observed in our patient, a superior prognosis was obtained in the present case.
Conclusions
We reported the case of a patient who showed a pCR after undergoing treatment with XELOX + Bev for synchronous resectable solitary liver metastasis from sigmoid colon cancer. The maximum diameter of the liver deposit in this case was 5.7 cm with a grade of H2 according to the Japanese classification. A pCR in a patient with H2 liver metastasis is considered rare based on a literature review.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Nordlinger B, Jaeck D, Guiguet M. eds. Surgical resection of hepatic metastases: multicentric retrospective study by the French Association of Surgery. Paris: Springer-Verlag, 1992.
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57; discussion 657-8.
- Jaeck D, Bachellier P, Nakano H, et al. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg 2003;185:221-9.
- Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 2004;240:1037-49; discussion 1049-51.
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16.
- Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol 2007;18:299-304.
- Blazer DG 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008;26:5344-51.
- Adam R, Wicherts DA, de Haas RJ, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol 2008;26:1635-41.
- Japanese Classfication of Colorectal Carcinoma (2nd English Edition). Tokyo, Japan: Kanehara & Co. Ltd, 2009.
- Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 2006;24:3939-45.
- Inoue M, Uehara K, Ishiguro S, et al. Pathologically complete response of multiple liver metastases from rectal cancer treated with mFOLFOX6 plus bevacizumab. Nihon Shokakibyo Gakkai Zasshi 2010;107:760-7.
- Klinger M, Tamandl D, Eipeldauer S, et al. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol 2010;17:2059-65.
- Gruenberger B, Tamandl D, Schueller J, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol 2008;26:1830-5.
- Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol 2011;22:2042-8.
- Gruenberger B, Scheithauer W, Punzengruber R, et al. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer 2008;8:120.
- Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118-24.
- Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007;110:2761-7.
- Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2010;17:2870-6.