Impact of cytolysis following transarterial chemoembolization for hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a frequent complication of liver disease. HCC is the sixth most common malignancy worldwide (1). Liver transplantation and resection are surgical therapies of curative intent under specific selection criteria (2-4). Unfortunately, many patients present with an advanced disease not amenable to surgical therapy. For these patients, locoregional therapies are the next best option. Transarterial chemoembolization (TACE) involves the delivery of a chemotherapeutic agent to the tumour via the hepatic artery. It is used in the treatment of large unresectable tumour (5,6), but also as a bridge therapy before liver transplantation (7) and to downstage a tumour to a size that is convenient for surgical management (8,9).
The post-chemoembolization syndrome (PCS), characterized by the elevation of blood transaminases accompanying right upper quadrant pain, nausea and fever is often observed after TACE. It manifests itself in the first few days after the treatment with a return to baseline transaminases levels after one week. The exact nature of liver cytolysis is controversial and while some have argued that it indicates tumour necrosis (10,11); for other it represents normal hepatocyte injury and a deleterious event (12,13). As most TACE protocols include a variety of analgesics, anti-inflammatory, anti-pyretic and anti-emetic agents that mask the symptoms associated with PCS, liver cytolysis is an objective sign of the syndrome’s occurrence.
At this moment, little is known on the short term impact of cytolysis occurring after TACE for hepatocellular cancer. In one study, neither post-chemoembolization syndrome nor cytolysis was associated with an improved tumour response 8 weeks after treatment (13). However, only half of the patients in the study had a diagnosis of hepatocellular cancer and cirrhosis was only present in forty percent.
In the present study, we investigate if the occurrence of cytolysis is associated with favourable radiological response in patients with hepatocellular carcinoma. Also, we will evaluate if the occurrence of cytolysis increases the risk of hepatic decompensation. Finally, we will look at the impact of cytolysis on overall survival after TACE.
Patients and methods
Data source
The study was conducted at the CHUM-Hôpital St-Luc (Montréal, Canada), a tertiary care center for hepatic diseases. The study was approved by our institutional board and the data was collected from the medical archives. Patients having received a chemoembolization treatment were identified using the Canadian Classification of Health Interventions (CCI) code 1.KE.51.GQ-M0 and the Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures (CCP) code 62.93. All charts identified were reviewed to verify that HCC was the underlying diagnosis. Data collected included demographics, underlying liver disorder, radiological evaluation of the tumor, chemoembolization protocol and laboratory tests. The date of death was obtained from the medical record. The number of lesions and diameter of the largest lesion targeted by the treatment, Model for End- Stage Liver Disease (MELD), Child-Pugh, Okuda and cancer of the liver italian program (CLIP) scores were calculated for all patients before each treatment. As Barcelona Clinic Liver Cancer (BCLC) staging classification was not yet standard at the time that several patients had their treatment, we didn’t have all the information to be able to use it as a prognostic score in this study. Values for aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, International Normalized Ratio (INR) were documented before and after treatment.
Patients
We identified a cohort of patients at our institution that had a TACE treatment between May 2005 and December 2009. Inclusion criteria were: age ≥18 years, diagnosis of HCC either proven by biopsy or supported by its radiological features according to American Association for the Study of Liver Disease (AASLD) guidelines valid at that time (14) and completion of a treatment of chemoembolization. Exclusion criteria were as follows: a tumour type other than HCC, simultaneous systemic treatment, embolization without chemotherapy, lack of information about lesion size prior to treatment, lack of biochemical data prior or within five days following treatment. Our cohort included patients for whom TACE had a curative or palliative intent.
Chemoembolization was performed by two radiologists (P.P, L.B) trained in interventional radiology and consisted in the infusion of a chemotherapeutic agent following selective catheterisation of a branch of the hepatic artery feeding the tumour. A larger territory was targeted when the tumour burden was extensive. At no moment treatment aimed both liver lobes Contraindications for the procedure at our institution are: Child-Pugh C, presence of portal vein thrombosis, presence of a large portovenal shunt, hepatofugal flow and presence of arterioportal fistulae (without prior coil embolization). The chemotherapeutic agent most often used was cisplatin mixed with lipiodol followed by a embolization with GelFoam particles (Pfizer, Canada). The dose of the chemotherapeutic agent was decided according to the size of the tumour to embolize.
Follow-up and response to treatment
Measurement of liver biochemistry was performed the day before the chemoembolization and then daily until discharge. At our institution, a contrast enhanced abdominal computed tomography (CT) scan or liver magnetic resonance imaging (MRI) with gadolinium are performed two months after TACE to evaluate the response to treatment. Accordingly, treatment can be repeated or the patient followed-up with a repeated imaging every 3 months if the initial response was satisfactory. Decision to repeat the treatment is taken in a multidisciplinary tumour board comprised of interventional radiologists, hepatobiliary surgeons and hepatologists. Radiological response was evaluated using the European Association for the Study of the Liver (EASL) criteria (15) and was judged favourable if either a complete (absence of enhancing tissue) or partial response (more than 50% reduction of enhancing tissue) was observed. Follow-up information was collected until the first of the ensuing events occurred: death of the patient, loss to follow-up, transplantation or hepatectomy.
Primary and secondary issues
The occurrence of cytolysis following chemoembolization was our main variable of interest. We used the definition by Paye et al. for cytolysis that is an elevation of AST above 100 UI/L with at least a doubling of the baseline value for AST (12) occurring within the first 5 days following treatment.
Our primary issue was to evaluate if cytolysis was associated with a favourable radiological response two months after treatment. Our secondary issues were to investigate if cytolysis was associated with the development of hepatobiliary complications and overall survival. Liver failure was defined as the development of hepatic encephalopathy, doubling of baseline bilirubin, 25% increase in INR or appearance of ascites during the hospitalisation post TACE.
Statistical analysis
The statistical analysis was twofold: first, we considered the treatment outcomes using the treatment as the unit of interest, as patients could undergo several sequential treatments. Second, we analysed the survival-related outcomes, this time using the patient as the unit of interest. When the unit of analysis was the treatment, generalized estimating equations (GEE) with an exchangeable correlation structure were used to account for the correlation between multiple treatments from the same patients. Continuous variables expressed as mean (standard deviation, sd) were compared with the Student t test when the unit of analysis was the patient. Similarly, categorical variables were compared using GEE at the treatment level and with the chi-square test at the patient level.
We constructed Kaplan-Meier curves for the time to death according to the presence or absence of cytolysis at the time of the first treatment. Cases were censored in case of transplantation or loss to follow-up. Administrative censoring was set at 18 months after the first treatment. Association among demographic, biochemical and prognostic score variables was estimated using multivariable Cox’s proportional hazards regression model. Variables selected were those that are known to be associated with survival from liver cancer and liver disease and included the alphafetoprotein (AFP) levels (16,17), Okuda score (18,19) or CLIP score (18,20,21), MELD score and patient’s age. The natural logarithm of the AFP was used for the analysis to improve the fit of the regression model.
To account for the impact of tumour differentiation on the response to chemotherapy, radiological response was adjusted according to the log of the alphafetoprotein levels as high AFP levels are associated with poorer tumour differentiation (22). Analyses were performed using R version 2.13.1 (The R Foundation for Statistical Computing, Vienna, Austria) statistical software and Stata v. 11.2 (StataCorp, College Station, Texas, USA). Odds and hazard ratios are provided with their 95% confidence interval.
Results
During the period from 2005 to 2009, we identified 176 patients from the medical archives that had a liver chemoembolization treatment. Nineteen patients were excluded (medical file missing: 1; two treatments at the same lesion within one week: 1; liver transplant within 5 days following TACE treatment: 1; missing transaminases values after treatment: 11; diagnosis other than hepatocellular carcinoma: 4; patient’s age <18 years old: 1). The average age was 63.4 years, 77% were males and 91.7% had a diagnosis of cirrhosis. Hepatitis C infection was the most common diagnosis. The 157 patients received a total of 280 treatments. Two treatments were excluded because there was no information about the lesion prior to treatment. Seven treatments lacked a radiological control after treatment (withdrawal of care 5, transplant 2) and were excluded from the radiological response but not the survival analyses. In total, 271 treatment cases were used to evaluate the radiological response. Cisplatin was the chemotherapeutic agent in 264 cases. Adriamycin and doxorubicin beads were used in the other 7 cases (6 and 1 respectively). Baseline characteristics according to the cytolysis status at the time of the first treatment are shown in Table 1. During follow-up, 29 (23%) patients in the cytolysis group had a liver transplant or a hepatectomy versus 3 (9.3%) in the non-cytolysis group. Twenty-two patients (17.6%) were lost to follow-up in the cytolysis group versus 4 (12.5%) in the no-cytolysis group. In both situations, the difference in proportions was not statistically significant. The overall incidence of cytolysis was 73% (198/271).
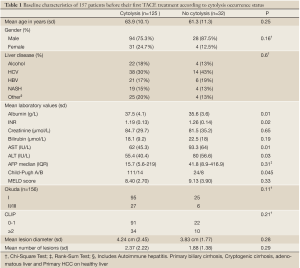
Full table
Radiological response
Response was analyzed using each treatment as the unit of analysis (n=271). After adjusting for the log(AFP), the odds-ratio (OR) estimate for cytolysis versus non-cytolysis was 1.90 (1.03-3.54), thus suggesting a favourable radiological outcome associated with cytolysis two months after treatment. The summary of the radiological response is shown in Table 2.
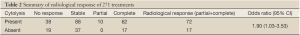
Full table
Effect of cytolysis on adverse events
Table 3 illustrates the adverse events observed after TACE treatment (n=271) according to cytolysis occurrence. There were 26 (14%) hepatobiliary complications in the cytolysis group and 5 (7%) in the non-cytolysis group. These included cases of hepatic encephalopathy, hyperbilirubinemia, coagulopathy (as defined in the methods section) and cholecystitis. There was a trend for a greater proportion of complications in the cytolysis group that was not statistically significant. No patient died or was listed for urgent transplant from liver failure complicating a TACE treatment (including the patient that was excluded from the analysis because of a transplant within 5 days of his treatment). No patient needed hemodialysis treatment from a renal complication. Hepatic complications from the treatment were transient with normalization of biochemical disturbances within a week.
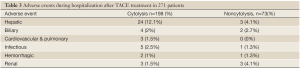
Full table
Overall survival
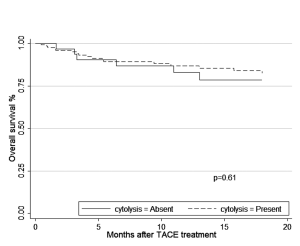
Survival was analyzed using as a start time the date that patients had their first TACE treatment and the unit of analysis was the patient (n=157). The differences of survival over time based on the presence of cytolysis are displayed in Kaplan-Meier curve (Figure 1). Our strategy for model selection took into account the limited number of death events. We restricted the number of variables in the model to include cytolysis, age, the AFP values, MELD score and a tumour prognostic score (CLIP or Okuda). After selection for the best model, the hazard ratio for survival comparing the patients with and without cytolysis after adjusting for age, pre-treatment AFP values, Okuda score and MELD score was 1.33 (0.45-3.90) (Table 4) .
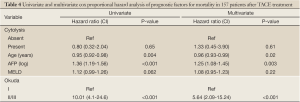
Full table
Predictors of cytolysis
Using a multivariate GEE model using the treatment as a unit of analysis (n=271), every increase in baseline AST values by one unit was associated with a decrease in the odds for cytolysis (OR 0.987; 0.975-0.999). Tumour size was not identified as an independent predictor for cytolysis within the same model (OR 1.136; 0.908-1.421).
Discussion
Originally, PCS was defined as the presence of fever, abdominal pain and vomiting during the first few days following TACE (23) and its incidence varies from 40-85% (13). Tumour size was a predictive factor for its occurrence (24). An early study by Castells et al. associated the incidence of fever to tumour necrosis and thus as an early marker of treatment response (11). Paye et al. redefined the post-chemoembolization syndrome as the presence of cytolysis (elevation of liver transaminases) associated with fever. His study failed to reveal an association between chemoembolization fever or cytolysis and tumour necrosis. PCS was more often observed in fibrotic rather than cirrhotic livers. The authors concluded that post-chemoembolization syndrome was a sign of normal hepatocyte destruction and not tumour necrosis (12). The association between fibrosis and cytolysis could have been confounded by tumour size as the tumours were significantly larger in the fibrotic compared to the cirrhotic livers. Using the same definition for post-chemoembolization syndrome, Wigmore et al. supported this conclusion in a subsequent investigation. In this study, 145 patients (75 with HCC and only 58 with cirrhosis) who underwent a TACE treatment were analyzed. A majority developed an elevation of transaminases (93%). Since transaminases are produced by hepatocytes or hepatocyte-derived tumour cell and the pattern of cytolysis was not determined by tumour type (primary liver tumour vs. secondary liver tumour), it was inferred that cytolysis was due to injury to the normal hepatocytes. Furthermore, neither post-chemoembolization syndrome nor cytolysis were associated with improvement in tumour response (13).
Our cohort was composed of patients who had hepatocellular cancer and, for a majority, underlying cirrhosis. Occurrence of cytolysis was associated with a 90% increase in odds of observing a radiological response to the treatment after adjusting for the baseline AFP levels. A reason why our results differ from the study by Wigmore et al. might be that half of their tumours were metastasis from adenocarcinomas. The vascular pattern differ in these two types of tumours and this allow radiologists to diagnose HCC only with imagery (14,25). The difference in the vascular behaviour may account for the dissimilar response to TACE.
Another important outcome from this study is the relative safety of TACE when patients are carefully selected. Occurrence of cytolysis was associated with a trend for an increased risk of hepatobiliary complications. However, none of the patients died as a consequence of TACE and the episodes of liver failure were transient. Other studies have shown similar results with cases of irreversible hepatic failure present in 3.1% (24), 3% (26) and no death in the postchemoembolization course (12). Therefore, if the perturbations in liver function are only transient, irreversible liver failure is rare and elevation of AST is not a predictor of an adverse hepatic outcome, we can question the necessity of daily liver chemistry measurements.
A recent study be Memon et al. showed an association between radiological response using the EASL criteria and improved survival at 6 and 12 months after TACE with a palliative intention (27). If cytolysis is associated with a better radiological response in our study, we would have expected to also observe an increase in survival. Our results are inconclusive in that cytolysis was associated with a 1.33 times higher hazard rate (HR) for overall survival in a multivariable model at 18 months after the first TACE treatment, but with a confidence interval that crossed the null. Our cohort included a mixture of patients that receive TACE for curative and palliative intent and that could explain why our results differ from Memon’s study. Overall, the number of deaths observed in our cohort were smaller than in other studies (16,19,28). It reflects a meticulous selection of patients with good baseline liver function and for whom TACE is not only used for palliation but also for a curative intent.
This study has several strengths. To the best of our knowledge, our cohort is the largest of the other studies looking at the effects of cytolysis on tumour response. Also, we only concentrated our study on hepatocellular carcinoma and excluded tumours that might have a different biological behaviour and prognosis such as neuroendocrine tumours, fibrolamellar subtype of hepatocarcinoma and less frequently metastatic adenocarcinomas. Our cohort was in a great majority composed of patients with cirrhosis, who are at highest risk for this type of cancer. Since all patients receiving a TACE treatment were hospitalized following their treatment, occurrence of cytolysis was properly assessed.
The study also has several limitations. It was a retrospective cohort study and we had to rely on the dictated radiological reports to assess radiological response, thus potentially leading to a misclassification of outcome. HCC are three-dimensional and an evaluation of tumour volume rather than diameter may not come with the same association between cytolysis and tumour response. However, three-dimensional measurement is not performed commonly outside of experimental trials and in common clinical practice, radiological response is evaluated in two-dimensions by a sole radiologist. We analyzed several biochemical and prognostic variables for confounding, but unidentified confounding is an issue in this type of studies. We could not evaluate the BCLC staging classification because it was not the standard at the time that the treatments were done. Our cohort had a survival rate that was higher than what we expected and the low number of events had an impact on the power of our study for survival analysis. Selection bias from the large proportion of losses to follow-up can complicate the interpretation of the study findings. Finally, the definition of cytolysis used in this study was the same as the one used in previous studies (12,13). This definition is arbitrary and not based on any biological criteria.
In conclusion our study showed that cytolysis after TACE in patients with hepatocellular carcinoma was associated with an improved radiological response, but not in overall survival up to 18 months after treatment. Furthermore, TACE is relatively safe in well selected patients with no cases associated with irreversible liver failure despite transient deterioration in liver function.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108.
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403.
- Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998;27:1578-83.
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003;9:557-63.
- Chapman WC, Majella Doyle MB, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 2008;248:617-25.
- Yao FY, Breitenstein S, Broelsch CE, et al. Does a patient qualify for liver transplantation after the down-staging of hepatocellular carcinoma? Liver Transpl 2011;17:S109-16.
- A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med 1995;332:1256-61.
- Castells A, Bruix J, Ayuso C, et al. Transarterial embolization for hepatocellular carcinoma. Antibiotic prophylaxis and clinical meaning of postembolization fever. J Hepatol 1995;22:410-5.
- Paye F, Farges O, Dahmane M, et al. Cytolysis following chemoembolization for hepatocellular carcinoma. Br J Surg 1999;86:176-80.
- Wigmore SJ, Redhead DN, Thomson BN, et al. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br J Cancer 2003;89:1423-7.
- Bruix J, Sherman M, Practice Guidelines Committee, et al. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36.
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30.
- Eltawil KM, Berry R, Abdolell M, et al. Analysis of survival predictors in a prospective cohort of patients undergoing transarterial chemoembolization for hepatocellular carcinoma in a single Canadian centre. HPB (Oxford) 2012;14:162-70.
- Tyson GL, Duan Z, Kramer JR, et al. Level of α-fetoprotein predicts mortality among patients with hepatitis C-related hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:989-94.
- Levy I, Sherman M, Liver Cancer Study Group of the University of Toronto. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut 2002;50:881-5.
- Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918-28.
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5.
- Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 2000;31:840-5.
- Li P, Wang SS, Liu H, et al. Elevated serum alpha fetoprotein levels promote pathological progression of hepatocellular carcinoma. World J Gastroenterol 2011;17:4563-71.
- Venook AP, Stagg RJ, Lewis BJ, et al. Chemoembolization for hepatocellular carcinoma. J Clin Oncol 1990;8:1108-14.
- Chung JW, Park JH, Han JK, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology 1996;198:33-40.
- Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2.
- Chan AO, Yuen MF, Hui CK, et al. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer 2002;94:1747-52.
- Memon K, Kulik L, Lewandowski RJ, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology 2011;141:526-35, 535.
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71.


