
Unexpected plasmablastic lymphoma in a young adult with unknown HIV infection: case report
Introduction
Plasmablastic lymphoma (PBL) is an extremely rare type of non-Hodgkin lymphoma (NHL), which accounts for less than 1% of all NHLs. The most frequent clinical scenario for PBL is an oral lesion in a patient with human immunodeficiency virus (HIV) infection. However, other types of immunodeficiency can also predispose individuals to the development of PBL, including iatrogenic forms (transplantation), but it has also been rarely reported in the elderly and immunocompetent individuals. PBL is frequently juxtaposed to the morphologic appearance of some diffuse large B cell lymphomas, plasmablastic/plasma cell myeloma (PCM), and Burkitt’s lymphoma, amongst others. However, the distinction between these entities is imperative due to its predilection for immunodeficient patients, aggressive clinical course, and poor overall prognosis. Because of the possibility for histologic overlap, the immunophenotypic pattern and appropriate testing for immunodeficiency etiologies, particularly HIV, and adequately acquiring relevant clinical/social history is extremely important for diagnosis, especially when presenting in a less familiar location in patients with minimal clinical history or unknown immunodeficiency (1,2). We hereby report a unique case of PBL in an unusual location with an unexpected clinical presentation as a perianal hemorrhoid/mass in a young adult male with no significant past medical history except a recently burst hemorrhoid. We present the following case in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-779/rc).
Case presentation
The patient was a 19-year-old young adult male with a past medical history only of recently developed external hemorrhoids that reportedly burst one week before presentation. Subsequently, he developed three days of perianal swelling and pain with decreased bowel movements. Prior to his presentation to the emergency department, no other interventions for this were attempted at home or in another facility. No other significant past medical, social, or family history was discovered then. Physical examination in the emergency department revealed a painful 8 cm indurated mass with flocculation at the posterior perianal region. Initial diagnostic consideration was most concerning for a sizeable perirectal abscess following hemorrhoid development. Due to the suspicion of infection, an incision and drainage was performed on the mass, which revealed extensive blood and necrotic tissue but no purulent material. The tissue was sent in formalin to pathology for routine analysis. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Pathological findings
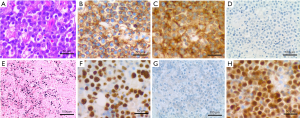
Histologic examination of the tissue reveals sheets of large plasmablastic cells with eccentrically located round nuclei with open chromatin, prominent nucleoli and abundant amount of cytoplasm. Frequent apoptotic bodies and mitotic figures are also present. Immunohistochemistry shows strong, diffuse positivity for leukocyte common antigen (CD45), CD10, plasmacytic markers (CD38, MUM1, CD79a), and negativity for B cell markers (CD20, PAX5), CD30, BCL2, BCL6, CD138, and spirochetes. The Ki-67 proliferation index is very high (>90%), and Epstein-Barr virus-encoded small RNA (EBER) in situ hybridization is diffusely positive (Figure 1). Molecular FISH testing for aggressive B cell lymphoma genetic abnormalities is positive for MYC gene rearrangement in 68% of the nuclei and the loss of 1 copy of the BCL2 gene on chromosome 18 in 64% of the nuclei. Follow-up FISH testing for B-cell lymphoma demonstrated t(8;14) with MYC/IGH fusion in 84% of nuclei without BCL2 or BCL6 rearrangement. Due to the absence of clinical suspicion for PBL or HIV, no HIV testing had been ordered. However, the morphologic and immunophenotypic findings prompted a request for additional clinical HIV testing, considering the frequent association between HIV and PBL, which subsequently returned positive. A final diagnosis of PBL was rendered.
Clinical management and follow-up
Initial CD4 (T helper) count was 321 mm3 with a T-helper percent of 19%. Staging was performed by follow-up bone marrow biopsy, abdomen and chest computed topography, and whole body positron emission tomography—computed tomography (PET/CT). The bone marrow biopsy showed no involvement at the biopsy site but the PET/CT demonstrated numerous sites of involvement, including the cervical, axillary, and inguinal lymph nodes and the perianal region. Therefore, due to lymphatic involvement above and below the diaphragm, in addition to extra-lymphatic involvement (perianal), he was staged as Stage IV using the Ann Arbor System. As a result of the extranodal involvement and diagnosis of stage IV, his National Comprehensive Cancer Network International Prognostic Index (NCCN-IPI) score was 2 (low-intermediate risk) and IPI score with age adjustment was 1 (low risk).
The patient was very concerned that his sexual preference for male partners would be discovered by his father, who he believed would not approve. Thus, he was initially skeptical of intense treatment but was explained the implications of his diagnosis and he subsequently agreed to therapy. The patient, in this case, was treated with V-EPOCH (Velcade/bortezomib, etoposide, prednisone, vincristine, doxorubicin, cyclophosphamide) for six cycles and intrathecal methotrexate for central nervous system prophylaxis for six cycles followed by autologous stem cell transplant (SCT). He was also initiated on antiretroviral therapy (ART). Adherence was reported as 100% based on routine follow-up office visits and patient self-reporting. The treatment was only complicated by chemotherapy induced thrombocytopenia, which resolved upon completion, and hyperuricemia, which was treated with Allopurinol. Following this treatment regimen, he was declared to be in complete remission. The patient expressed initial concern with receiving a bone marrow/SCT and immunosuppressive medications, but was ultimately understanding of the importance. Two and half years later, the patient has remained in remission while continuing ART. His CD4 count was 385 mm3 while his HIV PCR was 24 copies/mL on the most recent follow-up. There was no evidence of recurrence of the PBL at 3 years of follow-up as of today.
Discussion
The aggressive nature of PBL makes its accurate identification of paramount consequence as other lymphomas within the differential have varying treatment regimens. Furthermore, the testing for MYC genetic abnormalities is important as its presence portends a worse prognosis than in patients with normal MYC in some studies. The expression of CD45 has also been loosely associated with a better prognosis (1). Prognostic studies have revealed that swift diagnosis and subsequent treatment are just as important since overall survival without treatment is only three months for HIV-positive patients and slightly higher for HIV-negative patients at four months. Overall, the median survival for PBL is bleak at a median of just 6–11 months. Of note, resolution of PBL has been seen in patients with iatrogenically induced immunosuppression when their medications were reduced or held. However, this requires a case-by-case assessment for potential side effects. No definite standard of care has been developed for PBL due to the aggressive nature and poor prognosis. Routine CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) therapy is considered insufficient for this neoplasm; thus, more intensive combinations of EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, prednisone), CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine), and hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone/methotrexate, and cytarabine) have been used with no to only minimal benefit over CHOP. Intrathecal chemotherapy for neural prophylaxis is another consideration for this uncompromising neoplasm (1,3-5).
Other considerations include allogeneic SCT, which has shown promising results in some studies but only in those with HIV-negative PBL. Chimeric antigen receptor T-cell therapy (cART) has also been trialed in PBL but with mixed results. Radiotherapy is often considered as palliative care. Due to the plasmacytic differentiation seen in PBL, various myeloma drugs have been trialed, such as bortezomib, in combination with some of the regimens listed above (1,6,7). The dismal prognosis even with intensive treatment establishes the necessity for PBL specific therapies. Nonetheless, this may be a difficult task considering the rarity of the neoplasm, even amongst HIV-positive lymphomas.
Perianal lesions are relatively common amongst HIV+ individuals but are most often caused by infectious organisms instead of lymphoma. Further complicating the case and clinical presentation was the complete absence of prior infectious history and young age at presentation. This case of PBL is unique due to its presentation as a perianal “hemorrhoid” abscess in a young adult without known HIV infection and lack of prior clinical suspicion. Anal PBL is relatively uncommon, with only a handful of cases previously reported (3). Typically, this lymphoma presents as an oral lesion in an older patient (>40) with HIV. Not only was the presentation and location remarkable, but the immunohistochemical pattern also demonstrated slightly unusual results (CD138−, CD38+, MUM1+, CD45+, CD10+; Figure 1). In contrast, most PBLs are CD138+, CD38+/−, MUM1+, and CD45−, with a minority of cases showing positivity for CD10 as shown in this case (Table 1).
Table 1
| Positive | Negative |
|---|---|
| CD138 | CD45 (or weakly +) |
| CD38 | CD20 |
| MUM1/IRF4 | PAX5 |
| PRDM1 | Cyclin D1 |
| XBP1 | BCL2 |
| CD79a (~40%) | BCL6 |
| CD10 (20–40%) | |
| CD56 (~25% in extraoral sites) | |
| Cytoplasmic immunoglobin (kappa/lambda light chain and usually IgG) | |
| EMA | |
| CD30 | |
| Ki-67 (>70–90%) | |
| In-situ hybridization | |
| EBV small encoded RNA - EBER (In situ hybridization; ~75%) | |
| Genetic | |
| MYC translocation (~50% overall; ~73% in EBV+ cases) | |
| Clonal immunoglobin heavy chain gene rearrangement (IGH) |
Potential differentials to consider when diagnosing PBL include diffuse large B-cell lymphoma (DLBCL), plasma cell neoplasms (plasmablastic myeloma), anaplastic lymphoma kinase (ALK)+ large B cell lymphoma, Burkitt lymphoma, Castleman disease, and even melanoma or undifferentiated carcinoma. However, most of these can be relatively easily differentiated on pathological exam due to the immunohistochemical pattern. DLBCL and Burkitt lymphoma will show positivity for B-cell markers, including CD19, CD20, and PAX5. Positivity for CD45 may be useful in identifying DLBCL, but this marker was positive in this case of PBL. PCM can often be distinguished from PBL by morphology alone as PCM shows more typical plasma cell morphology without a blastic appearance and a markedly lower proliferation rate. These are also seldomly encountered in such young age patients. Multicentric Castleman disease is an HHV8 associated disorder that commonly presents as lymphadenopathy in patients with immunosuppression, including HIV infection. Multicentric Castleman disease can present histologically with plasmablasts that are infected with HHV8. However, these cases will also have the characteristic Castleman appearance with variably atretic germinal centers and prominent ‘onion skinning’ of the mantle zone. The plasmablasts in Castleman disease are often within the interfollicular region of the lymph nodes and show light-chain restriction (often Lambda) with variable CD20, CD38, CD79a, and vIL6 staining, but negativity for CD138, PAX5, and EBV. The classic penetrating venules may also be present. Finally, carcinomas can cause confusion due to CD138 positivity but are ultimately cytokeratin positive with negativity for MUM1 and CD38. Melanomas will show a variable reaction to common melanocytic markers (S100, HMB-45, Melan-A). Therefore, the most difficult differentials to consider are often plasmablastic PCM and ALK-positive large B-cell lymphoma (1-6).
In this case, a main differential diagnosis included Burkitt lymphoma considering the location, patient age, CD10 positivity, and MYC rearrangement with immunoglobin heavy chain (IGH). However, MYC rearrangement is relatively common amongst PBL, occurring in approximately 50% of cases overall and up to 74% in those positive for EBV. Furthermore, immunoglobin genes are the most frequent partners, including IGH. Nonetheless, the histologic features are typically quite different between the two entities with Burkitt lymphoma demonstrating a diffuse proliferation of medium-sized cells with multiple nucleoli, squared-off cell borders, and chromatin clumping while PBL typically has a prominent plasmablastic appearance with variable plasmacytic differentiation, which is not typical for Burkitt lymphoma. Another important differentiating factor from Burkitt lymphoma is the absence of B-cell markers CD20 and PAX5 in PBL. Although CD10 was positive in this case of PBL, BCL6, which is seen in Burkitt lymphoma, was negative. In the setting of immunodeficiency, such as HIV infection, EBV positivity is only seen in 25–40% of cases of Burkitt lymphoma. Plasmablastic PCM has significant overlap with PBL both histologically and immunophenotypically. Thus, differentiating between the two is based on clinical features and further ancillary studies that favor one. Plasmablastic PCM is more likely to have serum monoclonal paraproteinemia, lytic lesions on imaging with bone marrow involvement, renal dysfunction, hypercalcemia, and EBV negativity (EBER−). On the other hand, PBL is favored by EBV positivity and an extremely high mitotic index/proliferation rate on Ki-67 (>70–90%). ALK-positive large B-cell lymphoma is also remarkably rare with similar morphology and immunophenotype to that of PBL. However, ALK-positive large B-cell lymphoma will be positive for anaplastic ALK but will be negative for CD30, which can help to differentiate it from PBL (ALK−/CD30+). In addition, the rearrangement commonly seen in PBL (MYC at 8q24) is not seen in ALK-positive large B-cell lymphoma, which most often contains t(2;17)(p23;q23) (1,2,4-6).
Additional workup, in this case, showed EBER positivity and translocation 8;14 resulting in fusion of MYC and IGH. A subsequent request for clinical investigation revealed a positive HIV test in the patient. This further testing, combined with previously discussed findings, helped to confirm the diagnosis of PBL.
In summary, this case of PBL represents an unusual clinical presentation and a slightly unusual immunophenotype with pathologic findings that prompted testing and swift treatment of the patient’s HIV. This case demonstrates the importance of a timely tissue sampling with pathologic analysis along with thorough clinical and social investigations in patients with unusual presentations that are suggestive of immunodeficiency related neoplasms. Extra-oral PBL is uncommon, while the perianal location of PBL is exceedingly rare. The immunohistochemical pattern, in this case (CD45+, CD138−, CD38+, MUM1+, CD10+), is partially divergent from that typically seen in PBL (Table 1). The prognosis for PBL is dismal despite the availability of numerous intensive chemotherapy regimens, which highlights the importance of further research and treatment development more specific to this aggressive lymphoma. The treatment used in this patient proved successful thus far, but continued close follow-up will be necessary as only minimal evidence is available to guide treatment for this aggressive lesion and larger clinical trials with more cases may be needed for further investigation of the efficacy of this therapeutic approach. Another limitation for the current study is a somewhat brief follow-up time of approximately 3 years. However, considering the median survival for this disease, a follow-up time of this length seems acceptable.
Acknowledgments
The material had been partially presented as Poster form in America Society for Clinical Pathologists (ASCP) 2019 Annual Meeting, September 11-13, Phoenix, AZ, USA. [Am J Clin Pathol 2019;152:S110-S111. https://doi.org/10.1093/ajcp/aqz121.015 First published online: 11 September 2019].
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-779/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-779/coif). DD received financial support from the Department of Pathology at Loma Linda University Health for attending the ASCP National Meeting in September of 2019, where he presented a poster of this case report. He received no other outside funding for this presentation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harmon CM, Smith LB. Plasmablastic Lymphoma: A Review of Clinicopathologic Features and Differential Diagnosis. Arch Pathol Lab Med 2016;140:1074-8. [Crossref] [PubMed]
- Ahn JS, Okal R, Vos JA, et al. Plasmablastic lymphoma versus plasmablastic myeloma: an ongoing diagnostic dilemma. J Clin Pathol 2017;70:775-80. [Crossref] [PubMed]
- Isfahani F, Amar S, Dave H, et al. Plasmoblastic lymphoma as cause of perianal fistula: a case report and literature review. J Int Assoc Provid AIDS Care 2015;14:17-20. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer, 2017.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood 2015;125:2323-30. [Crossref] [PubMed]
- Koizumi Y, Uehira T, Ota Y, et al. Clinical and pathological aspects of human immunodeficiency virus-associated plasmablastic lymphoma: analysis of 24 cases. Int J Hematol 2016;104:669-81. [Crossref] [PubMed]
- Li YJ, Li JW, Chen KL, et al. HIV-negative plasmablastic lymphoma: report of 8 cases and a comprehensive review of 394 published cases. Blood Res 2020;55:49-56. [Crossref] [PubMed]


