
Resveratrol inhibits the proliferation, invasion, and migration, and induces the apoptosis of human gastric cancer cells through the MALAT1/miR-383-5p/DDIT4 signaling pathway
Introduction
Gastric cancer (GC) is the 4th most common malignant tumor in the world (1). Despite improvements in GC treatment methods, such as surgery, chemotherapy, and radiotherapy, these traditional therapies have a limited effect in reducing GC mortality (2). The invasion and metastasis of gastric cells are the major cause of most GC-related deaths and recurrences, and seriously hinder the therapeutic effects of any treatments (3). Thus, finding drugs that can effectively treat GC and inhibit tumor activity has become an urgent issue that needs to be addressed in the clinic.
Traditional cancer treatments are known to cause severe adverse reactions. Thus, in recent decades, research on the anti-cancer effect of phytochemicals has gradually become popular (4). Previous studies have found that chemical components in various natural plants have anti-cancer effects (5,6). Resveratrol (RS) is known to widely exist in grapes, peanuts, and other plants (7). At present, it has been found in breast cancer, ovarian cancer, liver cancer, lung cancer and other cancers that RS has various effects on inhibiting the growth, proliferation and invasion of cancer cells (1,5). Kim et al. found that RS inhibits the proliferation of GC cells by inhibiting the activity of Pim-1 kinase (1). Xu et al. found that RS inhibits GC epithelial-mesenchymal transformation and reverses Adriamycin resistance by regulating the phosphatase and tensin homolog/protein kinase B (PTEN/AKT) signaling pathway (7). However, there are few relevant literature reports on the inhibition of GC occurrence and development by RS, and there is a lack of specific mechanism research. With further research on the use of RS in the treatment of GC, explorations of the anti-cancer effect of RS have become crucial, and the results of such research provide a theoretical basis for its clinical application. Long non-coding RNAs (lncRNAs) are a series of transcribed RNA molecules with a length of more than 200 nucleotides, located in the nucleus or cytoplasm, and do not have the function of encoding proteins (8). A recent study suggested that lncRNA may be the molecular target of RS for its anticancer effect (9). In GC, it is known that MALAT1-mediated epithelial-mesenchymal transition inhibition of gastric cancer expression down-regulation or gene knockout may be related to the proliferation, migration and invasion of GC cells and the reduction of migratory ability and promotion of apoptosis (10). For example, Ji et al. found that RS triggers the metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)-mediated Wnt/β-catenin signaling pathway that inhibits the invasion and metastasis of colorectal cancer cells (11). Yang et al. found that RS inhibits the invasion and migration of GC cells by inhibiting epithelial-mesenchymal transformation mediated by MALAT1 (12).
At present, there are few studies on the role of RS and MALAT1 in GC. Thus, this study sought to examine the potential mechanism by which RS-mediated MALAT1 inhibits the proliferation, invasion, and migration of GC cells to provide theoretical support for the use of RS in the treatment of GC. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-307/rc).
Methods
Cell cultures
The human GC cell line SGC7901 was purchased from Shanghai Fuheng Biotechnology Co., Ltd. The cells were cultured in Roswell Park Memorial Institute Medium (1640) and placed in a 37 ℃ incubator (Thermo Fisher Scientific, USA) with 5% carbon dioxide. The cells were sub-cultured for subsequent experiments at 80% cell confluency.
Cell transfection
Micro ribonucleic acid–383-5p (miR-383-5p) inhibitor (inhibitor), negative control (NC), MALAT1-interfering RNA (si-MALAT1), si-deoxyribonucleic acid-damage-inducible transcript 4 (DDIT4) and negative interference control (si-NC) were obtained from Shanghai Jima Pharmaceutical Technology Co., Ltd. The specific sequences are as follows: siMALAT1 (sense strand: 5'-AAGAAAAAUAAAAGCUUUCCU-3', antisense strand: 5'-GAAAGCUUUUUAUUUUUCUUCC-3'), si-DDIT4 (sense strand: 5'-AGGAAGACACGGCTTACCT-3', antisense strand: 5'-GCTTCCGAGTCATCAAGAA-3'). Lipofectamine ™ 3000 (Invitrogen, USA) was used to transfect the cells, which were then cultured for 48 h.
Cell experiment grouping
- The effect of RS on MALAT1 level and proliferation activity of GC cells: The cells were divided into 3 groups: control group (Control), RS 1 µM group, and RS 5 µM group. The human gastric cancer cell line SGC7901 was treated with different concentrations of RS [0 (Control), 1, 5 µM] for 24 hours, respectively.
- Effects of RS treatment and interference with MALAT1 on the function of GC cells: The cells were divided into Control group, RS group (5 µM), si-NC group, si-MALAT1 group and RS+si-MALAT1 group. The groups that need to be transfected were treated accordingly, and then 5 µM RS was added to the cells in the RS+si-MALAT1 group for 24 hours.
- Effects of miR-383-5p and MALAT1 on the function of GC cells: cells were divided into Control group, si-NC + inhibitor NC group, si-MALAT1 + inhibitor NC group, si-NC + miR-383-5p inhibitor group, si-MALAT1 group + miR-383-5p group. Corresponding transfection treatments were performed on the cells in turn.
- Effects of miR-383-5p and DDIT4 on the function of GC cells: cells were divided into Control group, miR-383-5p group and si-DDIT4 + miR-383-5p inhibitor group. Corresponding transfection treatments were performed on the cells in turn.
Cell proliferation activity was measured by the CCK-8 method
The SGC7901 cells were incubated at the density of 104 per well in 96-well plates and treated the next day. After adding 10 µL of Cell Counting Kit 8 (CCK-8) solution (Dojindo, Japan) into each well, the plates were cultured for another 2 h and put into a multifunctional microplate reader to measure the absorbance value (OD) at a wavelength of 450 nm.
Apoptosis was measured by flow cytometry
The cells were digested with trypsin digestive solution without ethylenediaminetetraacetic acid, washed 3 times with pre-cooled phosphate buffer (PBS), and then centrifuged. The pellet was suspended with 500 µL of binding buffer. Next, 5 µL of annexin V-fluorescein isothiocyanate (FITC) (BD Biosciences, USA) and 5 µL of propidium iodide (BD Biosciences, USA) were added and dark stained at room temperature for 15 min. The apoptosis and apoptosis rate were immediately measured by flow cytometry (Thermo Scientific, USA) and Flowjo (TreeStar, USA) software, respectively.
Cell migration was measured by scratch tests
SGC7901 cells were seeded in 6-well plates at a density of 2×104 cells per well. When the cell confluency reached 90%, the tip of a pipette was used to draw 3 straight lines in parallel, and the fallen cells were then rinsed with 100 uL of PBS. The scratch healing was photographed under an inverted microscope (Olympus, Japan) at 0 and 24 h.
Cell invasion was detected by the transwell method
200 µL of cell suspension containing 1×104 cells was added to an upper chamber coated with matrix glue (BD Biosciences, USA), and 500 µL of normal culture medium was added to the lower chamber. The upper chamber was fixed with methanol at room temperature for 15 min. The cells that invaded the lower chamber were stained with 0.5% crystal violet (Beyotime, China) for 30 min. Pictures of transmembrane cells were taken under an inverted microscope, and the number of transmembrane cells was counted.
The protein expression of cells was detected by Western blot
Ristocetin-induced platelet aggregation cell lysis buffer (Beyotime, China) was used to extract the total cell protein, and the bicinchoninic acid assay method (Beyotime, China) was used to quantify it. The equivalent amount of protein was separated by 12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis and then transferred to the polyvinylidene fluoride membranes (Millipore, USA). After blocking the membrane with blocking solution (Beyotime, China) for 1 h, tris buffered saline with tween (TBST; Beyotime, China) was used to wash the membranes 3 times. The corresponding primary anti-deoxyribonucleic acid-damage-inducible transcript 4 (DDIT4, 1:2000, ab106356, Abcam, USA) and β-actin (1:3000, ab8226, Abcam, USA) was incubated overnight in a cold room. After washing the membranes with TBST 3 times, the corresponding secondary antibody combined with horseradish peroxidase was incubated at room temperature for 1 h. Next, an enhanced chemiluminescence detection kit (Beyotime, China) was used to visualize the protein bands. The Image J software (NIH, USA) was used to analyze the grayscale of the protein bands.
The gene transcription of cells was measured by fluorescence qRT-PCR
Trizol (Invitrogen, USA) was applied to lyse and extract the total RNA, and the RNA was quantified by a multifunctional enzyme labelling instrument (Thermo Scientific, USA). MALAT1 and miR-383-5p were synthesized into complementary DNA (cDNA) using a Prime Script kit (Takara, Japan) and a One Step Prime Script miRNA Synthesis Kit (Takara, Japan). Next, the cDNA was fluorescence amplified in the Applied Biosystems 7300 (Thermo Scientific, USA) system using SYBR green real-time polymerase chain assays (PCR) Master Mix (Thermo Scientific, USA), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or U6 as the internal reference gene, and the 2−ΔΔCt was used to detect the relative expression of genes. Multiple holes (3) were set for each sample. The following primer sequences were used: MALAT-1 front primer: 5'-GGTAACGATGGTGTCGAGGTC-3', rear primer: 5'-CCAGCATTACAGTTCTTGAACATG-3'. miR-383-5p front primer: 5'-GGGAGATCAGAAGGTGATTGTGGCT-3', rear primer: 5'-CAGTGCGTGTCGTGGAGT-3', U6 fronter prime: 5'-CTCGCTTCGGCAGCACA-3', and rear primer: 5'-AACGCTTCACGAATTTGCGT-3'.
The target was verified using the luciferase-reporter gene method
MALAT1 and DDIT4 fragments containing the miR-383-5p target sequence were amplified by quantitative real-time (qRT-PCR) and then inserted into pmirGlO double-luciferase miRNA target expression vector (Promega, USA). Wild-type MALAT1 (Wt-MALAT1) and Wt-DDIT4 reporter vectors were obtained. mutant MALAT1 (Mut-MALAT1) and mut-DDIT4 were constructed by inserting mutation binding sites. The Wt or Mut vectors and miR-383-5p mimic were co-transfected into the SGC7901 cells with Lipofectamine 3000.
Statistical analysis
All experiments in this study were independently performed 3 times. SPSS 22.0 (SPSS, USA) was used for the 1-way analysis of variance or the student’s t-test was used for the statistical analysis. A P value <0.05 was considered statistically significant.
Results
RS treatment decreases the MALAT1 level and proliferative activity of GC cells
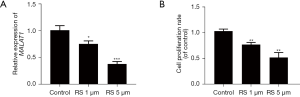
MALAT1 expression in the human GC cell line SGC7901 decreased as the RS dose increased [0 (Control), 1.5 µM)] (all P<0.05; see Figure 1A). Additionally, the cell proliferation activity was measured using the CCK-8 method, and the results showed that cell proliferation activity decreased as the RS dose increased (all P<0.05; see Figure 1B). The follow-up studies used 5 µM of the RS-treated cells.
Effects of RS treatment and interference with MALAT1 on the proliferation, migration, invasion, and apoptosis of GC cells
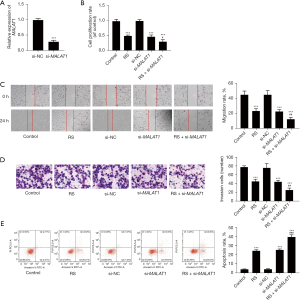
As Figure 2A shows, when the cells were transfected with si-MALAT1, the messenger RNA level of MALAT1 was significantly reduced (P<0.05), which provides evidence of the effectiveness of si-MALAT1. Cell proliferation was detected using the CCK-8 method. As Figure 2B shows, cell proliferation activity decreased significantly (all P<0.05) when the cells were administered 5 µM of RS or transfected with si-MALAT1. The inhibitory effect was more apparent when the cells received both treatments (P<0.05). In this study, the ability of cell migration and invasion was also measured. The results were consistent with those of the proliferation results (all P<0.05; see Figure 2C,2D). Additionally, as Figure 2E shows, flow cytometry was used to observe apoptosis. It was found that when RS or MALAT1 interference treatment was administered alone, the apoptosis rate increased significantly (all P<0.05), and when both were administered together, they promoted the occurrence of an apoptosis reaction (P<0.05).
MALAT1 targets miR-383-5p and inhibits its expression
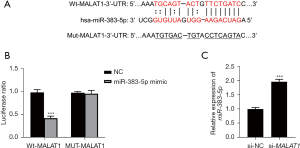
As Figure 3A shows, this study predicted that miR-383-5p was the target of MALAT1 through the Starbase website and then confirmed that MALAT1 targeted miR-383-5p through a luciferase-reporter gene analysis (P<0.05; see Figure 3B). Additionally, this study also detected the expression of miR-383-5p of the cells in the si-NC and si-MALAT1 groups. When the expression of MALAT1 decreased, the level of miR-383-5p increased significantly (P<0.05; see Figure 3C), which further confirmed the targeting relationship between them.
Interfering with miR-383-5p expression reverses the effects of interfering with MALAT1
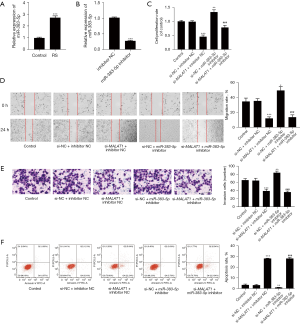
As Figure 4A shows, when RS was used to treat the GC cells, the expression level of miR-383-5p increased significantly (P<0.05). To verify the effectiveness of the miR-383-5p inhibitor, we detected the expression of the miR-383-5p in cells transfected with the NC inhibitor and miR-383-5p inhibitor, respectively. We found that the miR-383-5p inhibitor significantly reduced the expression level of miR-383-5p (P<0.05), which provides evidence of the effectiveness of the inhibitor (Figure 4B). Additionally, the reversal effect of the miR-383-5p inhibitor on si-MALAT1 was also measured when the cells were transfected with si-MALAT1 and miR-383-5p inhibitor at the same time. As Figure 4C-4F show, when the expression of miR-383-5p decreased, the cell proliferation activity increased (P<0.05), while the migration and invasion ability increased (all P<0.05), and apoptosis decreased (P<0.05). However, when si-MALAT1 and the miR-383-5p inhibitor were co-transfected, the above effects were reversed (all P<0.05). Thus, MALAT1 appears to regulate the proliferation, migration, invasion, and apoptosis of GC cells by targeting miR-383-5p.
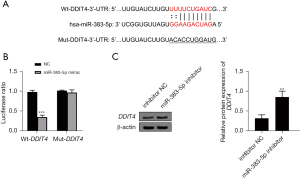
DDIT4 is the downstream target of miR-383-5p
As Figure 5A shows, this study predicted that DDIT4 was the target of miR-383-5p through the Starbase website. We then confirmed that miR-383-5p targeted DDIT4 through a luciferase-reporter gene analysis (P<0.05; see Figure 5B). Additionally, as Figure 5C shows, when the cells were transfected with the NC inhibitor or miR-383-5p inhibitor, the expression of miR-383-5p decreased significantly as the level of DDIT4 protein increased (P<0.05; see Figure 5C); thus, confirming the targeting relationship between them.
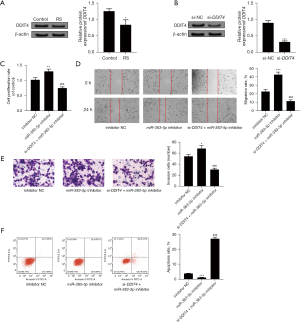
Interfering with DDIT4 expression in GC cells reverses the effects of the miR-383-5p inhibitor on cell proliferation, migration, invasion, and apoptosis
As Figure 6A shows, when RS was used to treat GC cells, the expression level of DDIT4 decreased significantly (P<0.05). To verify the effectiveness of interfering with DDIT4, we detected the expression of DDIT4 protein when cells were transfected with si-NC and si-DDIT4. We found that si-DDIT4 significantly inhibited the expression of DDIT4 protein (P<0.05), which provides evidence of the effectiveness of the interfering substance, as shown in Figure 6B. Additionally, the reversal effect of DDIT4 interference on the miR-383-5p inhibitor was also examined when cells were transfected with si-DDIT4 and miR-383-5p inhibitor at the same time. As Figure 6C-6F show, when the expression of miR-383-5p decreased, the cell proliferation activity increased (P<0.05), the ability of migration and invasion increased (all P<0.05), and the apoptosis decreased (P<0.05); however, when si-DDIT4 and miR-383-5p inhibitor were co-transfected, the above effects were reversed (all P<0.05). It is suggested that miR-383-5p can regulate GC cells’ proliferation, migration, invasion, and apoptosis by targeting DDIT4.
Discussion
According to China’s recent cancer statistics, GC is the 2nd leading cause of cancer-related death and poses a great threat to public health (13). Conventional chemotherapy or postoperative adjuvant chemotherapy is beneficial for GC patients, but some patients cannot bear the side effects of the treatment, which results in the interruption of treatment. Recently, a series of drugs have been developed in clinical trials to improve the treatment and prognosis of GC patients (14). RS has been widely studied because of its chemo-preventive and chemo-therapeutic potential (15), and research has shown that RS inhibits the proliferation of a variety of cancer cells (16,17). This study found that RS treatment significantly inhibits the proliferation, migration, and invasion of the GC cell line and induces apoptosis; thus, RS is a promising natural therapeutic drug for GC.
The function of MALAT1 has been confirmed in various human malignancies (18). The increased expression of MALAT1 promotes the proliferation of cancer cells in lung cancer (19). In pancreatic cancer, MALAT1 promotes the proliferation and invasion of cancer cells and other malignant behaviors (20). A previous study found that the RS treatment of GC cells inhibits the expression of MALAT1 (21). As a lncRNA, MALAT1 is known to play an important role in various cancers as a miRNA sponge, that is, as a potent miRNA inhibitor (22,23). Consistent with previous study, this study found that interfering with MALAT1 expression inhibits cell proliferation, invasion and migration, and promotes the occurrence of apoptosis (24). Thus, interfering with the expression of MALAT1 may become a potential therapy in the treatment of GC. Additionally, in view of the miRNA sponge function of MALAT1, through target prediction and a luciferase-reporter gene analysis, MALAT1 was found to target mir-383-5p and thus promote cancer. This suggests that MALAT1 may play its role in regulating cancer cell function by inhibiting the expression of miR-383-5p. A previous study shown that the expression of miR-383-5p in lung adenocarcinoma was significantly reduced (25). The overexpression of miR-383-5p in vitro inhibits cell proliferation and induces apoptosis through G1 cell-cycle arrest (25). In hepatocellular carcinoma, miR-383-5p has been shown to be a tumor suppressor that regulates the occurrence and development of hepatocellular carcinoma by targeting AKR1B10 (26). Azarbarzin et al. found that the expression of miR-383-5p is downregulated in intestinal GC, and can be used as a diagnostic biomarker (27). The present study found that RS treatment induces the increase of miR-383-5p expression in GC cells. When the expression of miR-383-5p decreases, proliferation, invasion, and migration increase, and the apoptotic response is inhibited. Further, when the expression of MALAT1 and miR-383-5p is reduced, the anti-cancer effect of miR-383-5p is inhibited, suggesting that MALAT1 plays a cancer promoting role by targeting miR-383-5p, that is, it plays a role in miRNA sponge adsorption.
Additionally, through target prediction and a luciferase-reporter gene analysis, we found that DDIT4 plays a role as the target of miR-383-5p. DDIT4 is induced by various stress conditions, including oxidative stress, endoplasmic reticulum stress, hypoxia, and hunger (28). In previous decades, the imbalance of DDIT4 expression has been observed in many human malignant tumors (28-30). For example, DDIT4 upregulates the CCAAT/enhancer-binding protein β mediated autophagosome-lysosome fusion and the desensitization of cells to anti-cancer drugs in prostate cancer cells (29). Additionally, baicalein has been shown to enhance DDIT4 levels and inhibit the proliferation of the mechanistic target of rapamycin complex 1 (mTORC1) and platinum-resistant cancer cells. Such results indicate that DDIT4 could potentially serve as a chemo-therapeutic and chemo-preventive agent (30). Du et al. previously reported that DDIT4 expression had a promotive effect in the proliferation and tumorigenesis of GC cells through the p53 and mitogen-activated protein kinase (MAPK) pathways (31). However, there is no relevant literature report on the regulation of DDIT4 by MALAT1 or miR-383-5p. In this study, through target prediction and luciferase reporter gene analysis, it was found that miR-383-5p could target DDIT4, thereby regulating its expression level. The present study found that a decrease in DDIT4 and miR-383-5p expression leads to the inhibition of the anti-cancer effect of miR-383-5p, which suggests that miR-383-5p may play an anticancer effect by targeting DDIT4 and regulating the expression level of DDIT4. Therefore, the above results show that the MALAT1/miR-383-5p/DDIT4 pathway may function as a potential pathway for the treatment of GC patients.
In conclusion, this study demonstrated that RS inhibits the proliferation, migration and invasion of human GC cells by regulating the MALAT1/miR-383-5p/DDIT4 pathway and induces apoptosis.
Acknowledgments
Funding: This work was supported by Zhejiang Medical and Health Research Project (Project No: 2019321913).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-307/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-307/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-307/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim S, Kim W, Kim DH, et al. Resveratrol suppresses gastric cancer cell proliferation and survival through inhibition of PIM-1 kinase activity. Arch Biochem Biophys 2020;689:108413. [Crossref] [PubMed]
- Machlowska J, Baj J, Sitarz M, et al. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci 2020;21:4012. [Crossref] [PubMed]
- Seeneevassen L, Bessède E, Mégraud F, et al. Gastric Cancer: Advances in Carcinogenesis Research and New Therapeutic Strategies. Int J Mol Sci 2021;22:3418. [Crossref] [PubMed]
- Kim WJ, Kim W, Bae JM, et al. Dehydroabietic Acid Is a Novel Survivin Inhibitor for Gastric Cancer. Plants (Basel) 2021;10:1047. [Crossref] [PubMed]
- Rauf A, Imran M, Butt MS, et al. Resveratrol as an anti-cancer agent: A review. Crit Rev Food Sci Nutr 2018;58:1428-47. [Crossref] [PubMed]
- Farvid MS, Holmes MD, Chen WY, et al. Postdiagnostic Fruit and Vegetable Consumption and Breast Cancer Survival: Prospective Analyses in the Nurses' Health Studies. Cancer Res 2020;80:5134-43. [Crossref] [PubMed]
- Xu J, Liu D, Niu H, et al. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res 2017;36:19. [Crossref] [PubMed]
- Wang C, Zhang Q, Hu Y, et al. Emerging role of long non-coding RNA MALAT1 in predicting clinical outcomes of patients with digestive system malignancies: A meta-analysis. Oncol Lett 2019;17:2159-70. [PubMed]
- Wang M, Jiang S, Yu F, et al. Noncoding RNAs as Molecular Targets of Resveratrol Underlying Its Anticancer Effects. J Agric Food Chem 2019;67:4709-19. [Crossref] [PubMed]
- Syllaios A, Moris D, Karachaliou GS, et al. Pathways and role of MALAT1 in esophageal and gastric cancer. Oncol Lett 2021;21:343. [Crossref] [PubMed]
- Ji Q, Liu X, Fu X, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One 2013;8:e78700. [Crossref] [PubMed]
- Yang Z, Xie Q, Chen Z, et al. Resveratrol suppresses the invasion and migration of human gastric cancer cells via inhibition of MALAT1-mediated epithelial-to-mesenchymal transition. Exp Ther Med 2019;17:1569-78. [PubMed]
- Thrift AP, Nguyen TH. Gastric Cancer Epidemiology. Gastrointest Endosc Clin N Am 2021;31:425-39. [Crossref] [PubMed]
- Wu J, Yu J, Wang J, et al. Astragalus polysaccharide enhanced antitumor effects of Apatinib in gastric cancer AGS cells by inhibiting AKT signalling pathway. Biomed Pharmacother 2018;100:176-83. [Crossref] [PubMed]
- Annaji M, Poudel I, Boddu SHS, et al. Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep (Hoboken) 2021;4:e1353. [PubMed]
- Selvaraj S, Sun Y, Sukumaran P, et al. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol Carcinog 2016;55:818-31. [Crossref] [PubMed]
- Kim TH, Park JH, Woo JS. Resveratrol induces cell death through ROS dependent downregulation of Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol Med Rep 2019;19:3353-60. [Crossref] [PubMed]
- Goyal B, Yadav SRM, Awasthee N, et al. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim Biophys Acta Rev Cancer 2021;1875:188502. [Crossref] [PubMed]
- Wang D, Zhang S, Zhao M, et al. LncRNA MALAT1 accelerates non-small cell lung cancer progression via regulating miR-185-5p/MDM4 axis. Cancer Med 2020;9:9138-49. [Crossref] [PubMed]
- Xu J, Xu W, Xuan Y, et al. Pancreatic Cancer Progression Is Regulated by IPO7/p53/LncRNA MALAT1/MiR-129-5p Positive Feedback Loop. Front Cell Dev Biol 2021;9:630262. [PubMed]
- Xu W, Ding M, Wang B, et al. Molecular Mechanism of the Canonical Oncogenic lncRNA MALAT1 in Gastric Cancer. Curr Med Chem 2021;28:8800-9. [Crossref] [PubMed]
- Zhang Z, Li M, Zhang Z. lncRNA MALAT1 modulates oxaliplatin resistance of gastric cancer via sponging miR-22-3p. Onco Targets Ther. 2020;13:1343-54. [Crossref] [PubMed]
- Li J, Gao J, Tian W, et al. Long non-coding RNA MALAT1 drives gastric cancer progression by regulating HMGB2 modulating the miR-1297. Cancer Cell Int 2017;17:44. [Crossref] [PubMed]
- Dai Q, Zhang T, Li C. LncRNA MALAT1 Regulates the Cell Proliferation and Cisplatin Resistance in Gastric Cancer via PI3K/AKT Pathway. Cancer Manag Res 2020;12:1929-39. [Crossref] [PubMed]
- Zhao S, Gao X, Zang S, et al. MicroRNA-383-5p acts as a prognostic marker and inhibitor of cell proliferation in lung adenocarcinoma by cancerous inhibitor of protein phosphatase 2A. Oncol Lett 2017;14:3573-9. [Crossref] [PubMed]
- Wang J, Zhou Y, Fei X, et al. Biostatistics mining associated method identifies AKR1B10 enhancing hepatocellular carcinoma cell growth and degenerated by miR-383-5p. Sci Rep 2018;8:11094. [Crossref] [PubMed]
- Azarbarzin S, Feizi MAH, Safaralizadeh R, et al. The Value of MiR-383, an Intronic MiRNA, as a Diagnostic and Prognostic Biomarker in Intestinal-Type Gastric Cancer. Biochem Genet 2017;55:244-52. [Crossref] [PubMed]
- Fattahi F, Saeednejad Zanjani L, Habibi Shams Z, et al. High expression of DNA damage-inducible transcript 4 (DDIT4) is associated with advanced pathological features in the patients with colorectal cancer. Sci Rep 2021;11:13626. [Crossref] [PubMed]
- Song L, Chen Z, Zhang M, et al. DDIT4 overexpression associates with poor prognosis in lung adenocarcinoma. J Cancer 2021;12:6422-8. [Crossref] [PubMed]
- Wang Y, Han E, Xing Q, et al. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett 2015;358:170-9. [Crossref] [PubMed]
- Du F, Sun L, Chu Y, et al. DDIT4 promotes gastric cancer proliferation and tumorigenesis through the p53 and MAPK pathways. Cancer Commun (Lond) 2018;38:45. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


