
Treatment paradigms and survival outcomes in esophageal adenocarcinoma with liver metastasis: a retrospective cohort study using the SEER database
Introduction
Esophageal cancer (EC) is one of the most common cancers worldwide (1). In 2020, 604,100 new cases of EC were reported, and EC was ranked as the 6th leading cause of death from cancer (1). Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are the two major histological types of EC (2). During the last few decades, the incidence of EAC has been rising rapidly, and it has become the most common esophageal malignancy in Western countries (3,4). According to a previous study, liver metastasis occurs in approximately 20% of EAC cases, and the liver is the most common organ of metastasis (5). With a median overall survival (OS) of 8–10 months and a 5-year OS rate <5%, the prognosis of EC patients is extremely poor (6).
Patients with esophageal adenocarcinoma with liver metastasis (EACLM) are classified as stage IVB under the tumor, node, metastasis (TNM) classification system, and are considered suitable for palliative therapy, such as chemotherapy (CT), palliative radiation therapy (RT), and salvage surgery (7-9). Systemic therapy can provide palliation of symptoms, improved survival, and enhanced quality of life in patients with locally advanced or metastatic esophageal or esophagogastric junction (EGJ) cancers (10-12). Radiation alone rarely cures EC, the combination of radiotherapy and concurrent CT has led to long-term survival approximately 25% of patients (13). Despite the increase in perioperative risks, the estimated 5-year survival of 25% has been reported in selected patients for salvage esophagectomy (2). However, the prognostic value of different treatment modalities for EACLM patients is not clear, with only a small number of retrospective case series have been described in the worldwide literature (7,14,15). Further, there is also a relative lack of knowledge about the prognostic factors for EACLM. Thus, the effects of optional treatment modalities on patients’ survival based on a large-scale cohort study urgently needed to be investigated.
This study sought to compare the OS and DSS of EACLM patients who were divided into the following groups: local therapy (surgery/radiation), systemic therapy (CT), combination therapy (surgery/radiation + CT), and no treatment using the Surveillance, Epidemiology and End Results (SEER) database. PSM analyses were performed to minimize the differences between the groups at baseline. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-420/rc).
Methods
Data source
The current study was a retrospective cohort study, and data about all the patients and their relevant information were obtained from the SEER database from 2010 to 2015. Follow-up data are obtained through active and passive methods. Data fields concerning patient vital status, date of last contact, treatment, and recurrence are updated to maintain accurate surveillance information. Patients were followed through the death date or the last follow-up date (December 31, 2018). The SEER database is an authoritative, public source of information on cancer incidence, mortality, prevalence, lifetime risk statistics, and survival in the United States (US) (16). We used SEER-Stat software (version 8.3.9) to access the database in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All data were extracted from the public database and did not involve personally identifiable information, so informed consent was not required.
Inclusion and exclusion criteria
According to the SEER database coding manual, all patients with histologic type, 8140/3, as coded by the International Classification of Diseases for Oncology (ICD-O-3), were included in the study. Patients were excluded from the study if they met any of the following exclusion criteria: (I) were not diagnosed at the first screening; (II) did not have liver-only metastases; (III) had missing treatment information; and (IV) had <1 month of follow-up (these patients were excluded to limit the immortal time bias) (17).
Study variables
Data and information were collected from the SEER database using SEER-Stat software for the following variables: age, gender, race, marital status, year of diagnosis, primary tumor site, T stage, N stage, tumor grade, treatment patterns, and survival time. Patients were divided into the following 4 groups based on age at diagnosis: 30–55, 56–65, 66–70, and >70 years old. Patients were divided into the following groups based on race: white, black, and other. Patients were divided into the following 4 groups based on marital status: separated/divorced, married, unmarried/single, and widowed/other. Patients were divided into the following 4 groups based on the distance from the incisors to the primary tumor site: 15–24 cm (C150–C153), 25–32 cm (C154), 33–40 cm (C155), and other (C158–159) (18). T stage and N stage were determined according to the American Joint Committee on Cancer (AJCC) (7th edition) staging system using the available clinical and pathologic data on tumor invasion, and lymph nodes status, respectively. Grade was defined by the following codes: well-differentiated (grade I), moderately differentiated (grade II), poorly differentiated or undifferentiated (grade III and grade IV), and unknown grade (other). Patients were divided into the following four groups based on treatment patterns: local therapy (surgery/radiation), systemic therapy (CT), combination therapy (surgery/radiation + CT), and no treatment.
Statistical analysis
Baseline
The baseline demographic and clinical characteristics are described as the medians with interquartile ranges (IQRs) for the continuous variables, and as the percentages for the categorical variables. The different groups (alive vs. dead) were compared using logistic regression models for all variables.
Univariate and multivariate regression analyses
For the univariate and multivariate regression analysis, the Cox regression model was used to assess the hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazards assumption was met. OS was used as the primary endpoint and defined as the time from diagnosis of EACLM to death from any cause. DSS was used as the secondary endpoint and referred to the period between diagnosis of EACLM and death due to EACLM. All the variables were included in the multivariate analysis to predict the independent prognosis factors, and the OS and DSS curves were examined using the Kaplan-Meier (K-M) method and compared using the log-rank test.
Propensity score-matching (PSM) analyses
PSM analyses were performed for sensitivity analysis. We used a PSM method to minimize the differences between the groups at baseline. A logistic regression model was conducted to evaluate the propensity score based on the following variables: age, gender, race, marital status, primary tumor site, T stage, N stage, and histological grade. A 1:1 PSM was implemented between patients with systemic therapy or no therapy, and their prognoses were also compared. We then further compared patients who underwent combination therapy and systemic therapy using a matching ratio of 1:2 to compare their prognoses. We used the nearest-neighbor matching algorithm based on the R package MatchIt and chose the caliper value at 10% of the standard deviation of the propensity score value as converted by the logit model. The standardized mean differences (SMD) before and after matching are illuminated in Figures S1,S2. The balance between datum line covariates in both the matched and unmatched cohorts was scanned by standardized differences, and <10% was adequately credible (19). After matching, the balance of variables between two groups was evaluated by the χ2 test and love-plot; a P value >0.05 for the χ2 tests or plots within two dashed vertical lines in the love-plot were considered balanced. R (version 4.0.5; https://www.r-project.org/) was used for the statistical analysis. A two-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 952 patients from 2010 to 2015 were identified as the study cohort from the SEER database. All the patients were confirmed to have EACLM at the time of the initial diagnosis. A flowchart of patient selection is presented in Figure 1. The median age of all patients at diagnosis was 63.0 (range, 30–97) years. Among these patients, the proportion of men was much greater (87.0%) than that of women (13.0%). Most patients (93.0%) were white; 4.0% were black, and 3.0% were other. Among the patients with EACLM, 24 (3%) of the 952 patients underwent surgery, 17 (2%) received RT, and 710 (75%) received CT. In relation to the various combination therapies, 26 (3%) of the 952 patients received combination therapy, 5 (1%) received local therapy, 685 (72%) received systemic therapy, and 236 (25%) did not received any treatment. Table 1 shows the baseline characteristics of all the patients extracted from the SEER database.
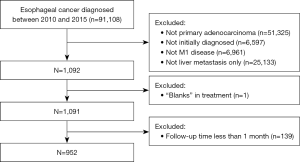
Table 1
| Variables | No. of patients [%] | P value |
|---|---|---|
| Age (years) | 0.504 | |
| 30–55 | 259 [27] | |
| 56–65 | 314 [33] | |
| 66–70 | 133 [14] | |
| 71–97 | 246 [26] | |
| Sex | 0.149 | |
| Female | 124 [13] | |
| Male | 828 [87] | |
| Race | 1.000 | |
| Black | 40 [4] | |
| White | 886 [93] | |
| Other | 26 [3] | |
| Marital status | 0.896 | |
| Married | 546 [57] | |
| Divorced/separated | 115 [12] | |
| Single/unmarried | 173 [18] | |
| Widowed/other | 118 [12] | |
| Year of diagnosis | <0.001* | |
| 2010 | 155 [16] | |
| 2011 | 148 [16] | |
| 2012 | 160 [17] | |
| 2013 | 141 [15] | |
| 2014 | 162 [17] | |
| 2015 | 186 [20] | |
| Tumor location (cm) | 0.280 | |
| 15–24 | 25 [3] | |
| 25–32 | 30 [3] | |
| 33–40 | 771 [81] | |
| Other | 126 [13] | |
| T stage | 0.164 | |
| T1 | 218 [23] | |
| T2 | 43 [5] | |
| T3 | 186 [20] | |
| T4 | 140 [15] | |
| Tx | 365 [38] | |
| N stage | 0.598 | |
| N0 | 261 [27] | |
| N1 | 453 [48] | |
| N2 | 69 [7] | |
| N3 | 44 [5] | |
| Nx | 125 [13] | |
| Grade | 0.02* | |
| I | 26 [3] | |
| II | 314 [33] | |
| III + IV | 464 [49] | |
| Other | 148 [16] | |
| Treatment | <0.001* | |
| Combination therapy | 26 [3] | |
| Local therapy | 5 [1] | |
| Systemic therapy | 685 [72] | |
| None | 236 [25] | |
| Surgery | 0.057 | |
| Yes | 24 [3] | |
| No | 928 [97] | |
| CT | <0.001* | |
| Yes | 710 [75] | |
| No | 242 [25] | |
| Radiation | 1.000 | |
| Yes | 17 [2] | |
| No | 935 [98] |
*, statistically significant. Percentages were calculated after excluding missing cases from the denominator. EACLM, esophageal adenocarcinoma with liver metastasis; CT, chemotherapy.
Survival analyses and prognostic factors
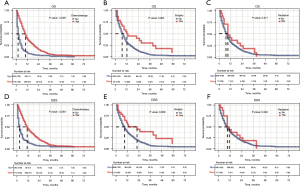
The median follow-up time of all 952 EACLM patients was 10.9 (range, 1–83) months. To investigate the relationship between treatment modality and prognosis, K-M survival analyses were conducted. The EACLM patients treated with CT had a better prognosis than those who were not treated with CT (P<0.001). Additionally, we found that the OS of patients who underwent surgery was longer than that of those who did not undergo surgery (P=0.005). However, there was no statistically significant different in the radiated patients compared to the non-radiated patients (P=0.271; see Figure 2A-2C). Similar results were also found in relation to DSS (see Figure 2D-2F). We further examined the effect of the treatment combinations on the OS and DSS of patients with EACLM. The median OS was 12, 10, 3, and 2 months for patients treated with combination therapy, systemic therapy, local therapy, and no therapy, respectively (see Figure 3A). Similar results were found for DSS. The median DSS was 19, 11, 3, and 3 months for patients treated with combination therapy, systemic therapy, local therapy, and no therapy, respectively (see Figure 3B).
In the univariate analyses, older age (71–97 vs. 30–55: HR =1.343, 95% CI: 1.119–1.611, P=0.002), year of diagnosis (2015 vs. 2010: HR =0.755, 95% CI: 0.597–0.954, P=0.019), T stage (T2 vs. T1: HR =0.640, 95% CI: 0.449–0.913, P=0.014; and T3 vs. T1: HR =0.798, 95% CI: 0.649–0.980, P=0.031), surgery (HR =0.528, 95% CI: 0.334–0.833, P=0.006), CT (HR =0.361, 95% CI: 0.310–0.420, P<0.001) were associated with OS. In the multivariate analyses, gender (HR =1.242, 95% CI: 1.055–1.535, P=0.045), T stage (T2 vs. T1: HR =0.583, 95% CI: 0.402–0.845, P=0.004; T3 vs. T1: HR =0.731, 95% CI: 0.586–0.912, P=0.006; and T4 vs. T1: HR =0.778, 95% CI: 0.613–0.987, P=0.038), and CT (HR =0.345, 95% CI: 0.292–0.407, P<0.001) were independent prognostic factors for OS (see Figure 4A).
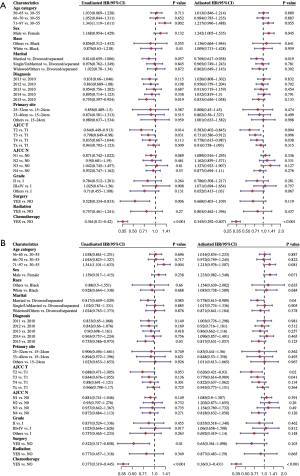
In relation to DSS, we also observed that factors such as older age (71–97 vs. 30–55: HR =1.341, 95% CI: 1.101–1.633, P=0.004), year of diagnosis (2015 vs. 2010: HR =0.755, 95% CI: 0.586–0.973, P=0.03), surgery (HR =0.522, 95% CI: 0.317–0.858, P=0.01), CT (HR =0.377, 95% CI: 0.319–0.445, P<0.001) were significant prognostic factors. The multivariate analysis of DSS indicated that marital status (married vs. divorced/separated: HR =0.778, 95% CI: 0.613–0.989, P=0.04), T stage (T2 vs. T1: HR =0.626, 95% CI: 0.421–0.930, P=0.02; T3 vs. T1: HR =0.779, 95% CI: 0.614–0.989, P=0.041), and CT (HR =0.360, 95% CI: 0.300–0.431, P<0.001) had significant predictive power compared to the other available factors (see Figure 4B).
PSM analyses
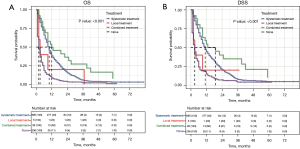
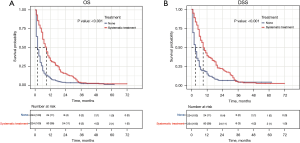
To better balance the patients in the systemic treatment group and the no therapy group, we performed a 1:1 PSM analysis for variables to decrease the selection bias and further compared their OS and DSS using the Cox regression model. The PSM analysis generated 224 matched pairs with similar baseline characteristics (see Table 2 and Figure S1). The results showed that patients who received systemic treatment demonstrated a better OS and DSS than those who did not received any therapy (9 vs. 2 months, P<0.001 and 9 vs. 3 months, P<0.001; see Figure 5).
Table 2
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| No treatment (n=236) | Systemic therapy (n=685) | P value | No treatment (n=224) | Systemic therapy (n=224) | P value | ||
| Age (years), n [%] | <0.001* | 0.577 | |||||
| 30–55 | 37 [16] | 213 [31] | 37 [17] | 46 [21] | |||
| 56–65 | 62 [26] | 241 [35] | 62 [28] | 53 [24] | |||
| 66–70 | 38 [16] | 93 [14] | 37 [17] | 41 [18] | |||
| 71–97 | 99 [42] | 138 [20] | 88 [39] | 84 [38] | |||
| Sex, n [%] | 0.783 | 0.893 | |||||
| Female | 33 [14] | 89 [13] | 31 [14] | 33 [15] | |||
| Male | 203 [86] | 596 [87] | 193 [86] | 191 [85] | |||
| Race, n [%] | 0.41 | 0.478 | |||||
| Black | 13 [6] | 26 [4] | 10 [4] | 14 [6] | |||
| White | 218 [92] | 638 [93] | 209 [93] | 202 [90] | |||
| Other | 5 [2] | 21 [3] | 5 [2] | 8 [4] | |||
| Marital status, n [%] | <0.001* | 0.924 | |||||
| Married | 115 [49] | 409 [60] | 113 [50] | 107 [48] | |||
| Divorced/separated | 30 [13] | 83 [12] | 30 [13] | 29 [13] | |||
| Single/unmarried | 44 [19] | 124 [18] | 41 [18] | 44 [20] | |||
| Widowed/other | 47 [20] | 69 [10] | 40 [18] | 44 [20] | |||
| Year of diagnosis, n [%] | 0.053 | 0.264 | |||||
| 2010 | 50 [21] | 102 [15] | 49 [22] | 46 [21] | |||
| 2011 | 28 [12] | 116 [17] | 26 [12] | 42 [19] | |||
| 2012 | 39 [17] | 113 [16] | 37 [17] | 31 [14] | |||
| 2013 | 42 [18] | 92 [13] | 40 [18] | 29 [13] | |||
| 2014 | 35 [15] | 122 [18] | 33 [15] | 32 [14] | |||
| 2015 | 42 [18] | 140 [20] | 39 [17] | 44 [20] | |||
| Tumor location (cm), n [%] | 0.205 | 0.677 | |||||
| 15–24 | 10 [4] | 14 [2] | 9 [4] | 5 [2] | |||
| 25–32 | 8 [3] | 20 [3] | 7 [3] | 9 [4] | |||
| 33–40 | 183 [78] | 565 [82] | 174 [78] | 173 [77] | |||
| Other | 35 [15] | 86 13] | 34 [15] | 37 [17] | |||
| T stage, n [%] | 0.266 | 0.905 | |||||
| T1 | 58 [25] | 153 [22] | 56 [25] | 49 [22] | |||
| T2 | 10 [4] | 32 [5] | 10 [4] | 9 [4] | |||
| T3 | 33 [14] | 138 [20] | 33 [15] | 36 [16] | |||
| T4 | 34 [14] | 103 [15] | 30 [13] | 35 [16] | |||
| Tx | 101 [43] | 259 [38] | 95 [42] | 95 [42] | |||
| N stage, n [%] | <0.001* | 0.516 | |||||
| N0 | 89 [38] | 166 [21] | 82 [37] | 68 [30] | |||
| N1 | 88 [37] | 349 [54] | 86 [38] | 90 [40] | |||
| N2 | 12 [5] | 53 [8] | 12 [5] | 19 [8] | |||
| N3 | 12 [5] | 29 [4] | 12 [5] | 11 [5] | |||
| Nx | 35 [15] | 88 [13] | 32 [14] | 36 [16] | |||
| Grade, n [%] | 0.185 | 0.810 | |||||
| I | 9 [4] | 16 [2] | 9 [4] | 11 [5] | |||
| II | 85 [36] | 213 [31] | 76 [34] | 78 [35] | |||
| III + IV | 103 [44] | 350 [51] | 103 [46] | 94 [42] | |||
| Other | 39 [17] | 106 [15] | 36 [16] | 41 [18] | |||
*, statistically significant. Percentages were calculated after excluding missing cases from the denominator. PSM, propensity score-matching.
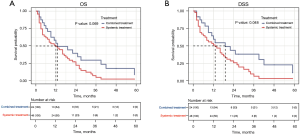
To determine if the combination therapy was superior to systemic therapy, we conducted a PSM analysis to assemble cohorts of patients with similar baseline characteristics and thereby reduced the possible bias in estimating treatment effects. Following 2:1 matching by propensity score, 24 patients in the combination therapy group were matched to 48 patients in the systemic therapy group. The baseline characteristics were well balanced between the two groups in both cohorts (see Table 3 and Figure S2). The results demonstrated that combination therapy did not improve the OS rate compared to systemic therapy (13 vs. 12 months, P=0.069; see Figure 6A). However, patients who received combination therapy had better DSS than those who received systemic therapy (19 vs. 13 months, P=0.048; see Figure 6B).
Table 3
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| Combination therapy (n=236) | Systemic therapy (n=685) | P value | Combination therapy (n=224) | Systemic therapy (n=224) | P value | ||
| Age (years), n [%] | 0.863 | 0.946 | |||||
| 30–55 | 9 [35] | 213 [31] | 8 [33] | 14 [29] | |||
| 56–65 | 9 [35] | 241 [35] | 8 [33] | 20 [42] | |||
| 66–70 | 2 [8] | 93 [14] | 2 [8] | 4 [8] | |||
| 71–97 | 6 [23] | 138 [20] | 6 [25] | 10 [21] | |||
| Sex, n [%] | 0.234 | 1.000 | |||||
| Female | 1 [4] | 89 [13] | 1 [4] | 3 [6] | |||
| Male | 25 [96] | 596 [87] | 23 [96] | 45 [94] | |||
| Race, n [%] | 0.827 | 0.232 | |||||
| Black | 0 [0] | 26 [4] | 0 [0] | 3 [6] | |||
| White | 26 [100] | 638 [93] | 24 [100] | 41 [85] | |||
| Other | 0 [0] | 21 [3] | 0 [0] | 4 [8] | |||
| Marital status, n [%] | 0.464 | 0.490 | |||||
| Married | 19 [73] | 409 [60] | 17 [71] | 40 [83] | |||
| Divorced/separated | 1 [4] | 83 [12] | 1 [4] | 1 [2] | |||
| Single/unmarried | 5 [19] | 124 [18] | 5 [21] | 5 [10] | |||
| Widowed/other | 1 [4] | 69 [10] | 1 [4] | 2 [4] | |||
| Year of diagnosis, n [%] | 0.428 | 0.992 | |||||
| 2010 | 3 [12] | 102 [15] | 3 [12] | 5 [10] | |||
| 2011 | 3 [12] | 116 [17] | 3 [12] | 8 [17] | |||
| 2012 | 8 [31] | 113 [16] | 7 [27] | 12 [25] | |||
| 2013 | 5 [19] | 92 [13] | 5 [21] | 10 [21] | |||
| 2014 | 4 [15] | 122 [18] | 4 [17] | 10 [21] | |||
| 2015 | 3 [12] | 140 [20] | 2 [8] | 3 [6] | |||
| Tumor location (cm), n [%] | 0.280 | 0.677 | |||||
| 15–24 | 1 [4] | 14 [2] | 1 [4] | 2 [4] | |||
| 25–32 | 2 [8] | 20 [3] | |||||
| 33–40 | 20 [77] | 565 [82] | 20 [83] | 40 [83] | |||
| Other | 3 [12] | 86 [13] | 3 [12] | 6 [12] | |||
| T stage, n [%] | <0.001* | 0.016* | |||||
| T1 | 6 [23] | 153 [22] | 6 [25] | 13 [27] | |||
| T2 | 0 [0] | 32 [5] | 0 [0] | 2 [4] | |||
| T3 | 15 [58] | 138 [20] | 14 [58] | 10 [21] | |||
| T4 | 2 [8] | 103 [15] | 2 [8] | 8 [17] | |||
| Tx | 3 [12] | 259 [38] | 2 [8] | 15 [31] | |||
| N stage, n [%] | <0.061 | 0.255 | |||||
| N0 | 3 [12] | 166 [21] | 3 [12] | 13 [27] | |||
| N1 | 15 [58] | 349 [54] | 13 [54] | 20 [42] | |||
| N2 | 4 [15] | 53 [8] | 4 [17] | 6 [12] | |||
| N3 | 3 [12] | 29 [4] | 3 [12] | 2 [4] | |||
| Nx | 1 [4] | 88 [13] | 1 [4] | 7 [15] | |||
| Grade, n [%] | 0.026* | 0.002* | |||||
| I | 0 [0] | 16 [2] | |||||
| II | 16 [62] | 213 [31] | 15 [62] | 10 [21] | |||
| III + IV | 8 [31] | 350 [51] | 7 [29] | 24 [50] | |||
| Other | 2 [8] | 106 [15] | 2 [8] | 14 [29] | |||
*, statistically significant. Percentages were calculated after excluding missing cases from the denominator. PSM, propensity score-matching.
Discussion
The liver is the most common metastatic organ in patients with EAC, and such patients have very poor outcomes (20). In this large cohort study, we explored the therapeutic modalities and survival outcomes of patients with EACLM. We found that while the use of systemic therapy was the highest, the use of local and combination therapy was very low. Additionally, we also found that a substantial proportion of the patients did not receive any treatment, which was most likely due to their nutritional insufficiency and performance status. Based on our findings, CT remains the main treatment modality for patients with EACLM. Regardless of whether the patients received surgery or radiotherapy, patients treated with CT had a better OS and DSS. Our findings are consistent with the current National Comprehensive Cancer Network guidelines that recommend systemic therapy for patients with metastatic EAC to palliate symptoms, improve survival, and enhance patients’ quality of life (21).
Local therapy, including surgery or RT after effective systemic therapy, could reduce the tumor burden but their potential value in metastatic EC (mEC) remains controversial (9). Wu et al. analysed the OS of patients with mEC who were treated with local therapy, and found that patients who received preoperative RT had significantly better OS than patients who underwent primary surgery alone and postoperative RT (P<0.001) (9). In another study, Tanaka et al., found that there was no difference in survival between patients who underwent surgery and those who did not undego surgery (P=0.1291) (6). In our study, the multivariate survival analysis indicated that either surgery or RT was associated with a survival benefit. Thus, we are of the view that simple local therapy is ineffective and should not be recommended to patients with EACLM.
In the era of personalized treatment, a comprehensive multidisciplinary approach is widely applied to determine the optimal treatment for patients with locally advanced primary EC; however, its role is not well defined for patients with mEC (22). Previous research has shown that the survival of stage IVB EC patients with distant metastasis treated with multimodality therapy was significantly better than that of patients treated with single-modality therapy or best supportive care alone (P<0.0001) (6). Shao et al. reported that there was no statistically significant difference between the CT group and chemoradiotherapy (CRT) group in terms of OS and cancer-specific survival (CSS) in mEC patients. Further, their subgroup analyses revealed that EAC patients who underwent CT had a favorable prognosis (8). In another study, Qiu et al. examined elderly stage IVB EAC patients with distant metastasis, and reported that compared to untreated patients, patients treated with surgery, RT, and CT had a better prognosis (OS and CSS: P<0.001) (20). Our PSM analyses showed that patients treated with systemic therapy had a much better prognosis in terms of OS and DSS than those who were untreated. Additionally, there was no significant difference in terms of OS between the patients who received combination therapy and systemic therapy. However, combination therapy had survival advantages in terms of the DSS of patients with EACLM. As local therapy (either surgery or RT) is inevitably accompanied by some treatment-associated complications (22,23), we suggest that combination therapy be considered for patients with EACLM after a comprehensive assessment by a multidisciplinary team (24).
The prognostic factors for EACLM were investigated by Cox regression analyses. The results revealed that gender, T stage, and CT were powerful and independent prognostic factors for OS, while marital status, T stage, and CT were independent prognostic factors for DSS. According to Tang et al., factors, including age, gender, grade at diagnosis, the number of metastatic organs at diagnosis, pathological type, local treatment, and CT, were independent predictors of CSS for patients with stage IV esophageal carcinoma (25). In line with a previous study (26), we found that married patients had better DSS than unmarried patients. A major reason for this is that married patients tend to choose positive treatment and demonstrate better compliance than unmarried patients, which may produce better survival advantages (27).
Our study revealed that age is not an independent prognostic factor for EACLM patients. Previous studies have drawn inconsistent conclusions about the relationship between age and prognostic risk in mEC patients (5,25). However, one such study did not distinguish between the histology types of EAC and ESCC, while another study did not indicate which type of organ metastasis was more likely to occur in younger patients. The identification of the prognostic factors associated with the patients would help in the prognostication and management of EACLM.
We undertook a comprehensive analysis of the treatment patterns and the survival outcomes of EACLM patients; however, this study still had some limitations. First, the data obtained from the SEER database lacked some important information, including information about the radiation dose, quality of life, and CT drug regimens, which may have led to an immortal time bias. Second, all the patients examined in this study were from the US; thus, the results do not represent the global population. Finally, as immune checkpoint inhibitors, such as programmed cell death protein 1, and programmed death-ligand 1, are currently being developed and examined, the prognosis of patients will certainly improve. Thus, it is necessary to provide updated information using such data.
Conclusions
This is a large-scale report on the treatment patterns and prognosis of patients with EACLM. CT-based combination therapy may be the most effective treatment strategy for such patients. The findings need to be externally validated in the future, but they may be useful in guiding clinical decision making, directing individualized treatment strategies, designing clinical trials, and ultimately improving patient prognosis.
Acknowledgments
Funding: This work was supported by the Nature Science Foundation of Ningbo (No. 2021J261).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-420/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-420/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Dubecz A, Solymosi N, Stadlhuber RJ, et al. Does the Incidence of Adenocarcinoma of the Esophagus and Gastric Cardia Continue to Rise in the Twenty-First Century?-a SEER Database Analysis. J Gastrointest Surg 2013; Epub ahead of print. [Crossref] [PubMed]
- Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012;23:3155-62. [Crossref] [PubMed]
- Ai D, Zhu H, Ren W, et al. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis 2017;9:3023-30. [Crossref] [PubMed]
- Tanaka T, Fujita H, Matono S, et al. Outcomes of multimodality therapy for stage IVB esophageal cancer with distant organ metastasis (M1-Org). Dis Esophagus 2010;23:646-51. [Crossref] [PubMed]
- Huddy JR, Thomas RL, Worthington TR, et al. Liver metastases from esophageal carcinoma: is there a role for surgical resection? Dis Esophagus 2015;28:483-7. [Crossref] [PubMed]
- Shao Y, Zhang M, Ye L, et al. Survival differences between chemotherapy and chemoradiotherapy in metastatic esophageal cancer: a propensity score-matched study based on the SEER database. Ann Palliat Med 2021;10:3826-35. [Crossref] [PubMed]
- Wu SG, Xie WH, Zhang ZQ, et al. Surgery Combined with Radiotherapy Improved Survival in Metastatic Esophageal Cancer in a Surveillance Epidemiology and End Results Population-based Study. Sci Rep 2016;6:28280. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. [Crossref] [PubMed]
- Janmaat VT, Steyerberg EW, van der Gaast A, et al. Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev 2017;11:CD004063. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Iitaka D, Shiozaki A, Fujiwara H, et al. Case involving long-term survival after esophageal cancer with liver and lung metastases treated by multidisciplinary therapy: report of a case. Surg Today 2013;43:556-61. [Crossref] [PubMed]
- Mudan SS, Giakoustidis A, Giakoustidis D, et al. Synchronous oesophagectomy and hepatic resection for metastatic oesophageal cancer: report of a case. Hippokratia 2010;14:291-3. [PubMed]
- Duggan MA, Anderson WF, Altekruse S, et al. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol 2016;40:e94-e102. [Crossref] [PubMed]
- Park HS, Gross CP, Makarov DV, et al. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1365-73. [Crossref] [PubMed]
- Gharzai L, Verma V, Denniston KA, et al. Radiation Therapy and Cardiac Death in Long-Term Survivors of Esophageal Cancer: An Analysis of the Surveillance, Epidemiology, and End Result Database. PLoS One 2016;11:e0158916. [Crossref] [PubMed]
- Zhao QY, Luo JC, Su Y, et al. Propensity score matching with R: conventional methods and new features. Ann Transl Med 2021;9:812. [Crossref] [PubMed]
- Qiu G, Zhang H, Wang F, et al. Metastasis Patterns and Prognosis of Elderly Patients With Esophageal Adenocarcinoma in Stage IVB: A Population-Based Study. Front Oncol 2021;11:625720. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Paryani NN, Ko S, Hobbs CJ, et al. Multimodality therapy for locally advanced esophageal cancer. J Clin Oncol 2015;33:abstr 211.
- Raymond D. Complications of esophagectomy. Surg Clin North Am 2012;92:1299-313. [Crossref] [PubMed]
- Zhang S, Guo J, Zhang H, et al. Metastasis pattern and prognosis in men with esophageal cancer patients: A SEER-based study. Medicine (Baltimore) 2021;100:e26496. [Crossref] [PubMed]
- Tang X, Zhou X, Li Y, et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Patients with Initially Diagnosed Metastatic Esophageal Cancer: A SEER-Based Study. Ann Surg Oncol 2019;26:321-8. [Crossref] [PubMed]
- Patel MI, Schupp CW, Gomez SL, et al. How do social factors explain outcomes in non-small-cell lung cancer among Hispanics in California? Explaining the Hispanic paradox. J Clin Oncol 2013;31:3572-8. [Crossref] [PubMed]
- Xiong Y, Shi X, Hu Q, et al. A Nomogram for Predicting Survival in Patients With Breast Cancer Liver Metastasis: A Population-Based Study. Front Oncol 2021;11:600768. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


