
The efficacy and safety of continuous intra-arterial infusion neoadjuvant chemotherapy with surgery for locally advanced gastric cancer: a preliminary pilot study
Introduction
Gastric cancer (GC) is one of the leading causes of malignancy-related morbidity and mortality worldwide (1), and especially in East Asia. Most GC patients are already at an advanced stage when diagnosed (2). Although surgical resection is the only curative treatment for GC, many patients with advanced disease fail to achieve R0 resection after surgery alone. Preoperative or neoadjuvant chemotherapy (NAC) has been regarded as the standard of care for patients with locally advanced GC, especially in terms of tumor downstaging, improved radical (R0) resection, and survival outcomes (3,4). Previous studies of preoperative systemic chemotherapy have reported a complete or subtotal pathological response rate of 3.3–37%, and an overall pathologic response rate of 42.2–89.7% (5-9). Various chemotherapy regimens and routines have been studied for the perioperative treatment of patients with advanced GC. However, due to the inaccuracy of chemotherapeutic drug delivery and the inability to maintain local high drug concentration, chemotherapy response remained unsatisfactory. The development of more effective and less toxic treatment options for GC remains a priority to enhance the surgical opportunities and survival outcomes of patients.
Intra-arterial infusion chemotherapy (CAIC) can increase the local drug concentration around the tumor sites, thereby offering potentially greater efficacy and less toxicity. Chemotherapy administered via intra-arterial infusion has been reported to result in a 20–30% higher response rate in advanced GC patients when compared with systemic chemotherapy (10). Regional arterial infusion chemotherapy (RAIC) is a form of CAIC in GC that involves injecting chemotherapeutic drugs directly into the tumor site via the tumor-feeding artery. Compared with systematic chemotherapy, RAIC can improve the tumor response and reduce the toxicity of drugs (11). Unlike RAIC, CAIC involves direct and continuous delivery of chemotherapeutic drugs into the tumor site through a tumor-feeding artery for a longer period of time. CAIC has been reported to provide survival benefits in patients with advanced hepatic cancer (12). However, few studies have been carried out with respect to neoadjuvant CAIC in GC. Also, as a useful tool for intraoperative navigation, C-arm computed tomography angiography (CACTA) has been used to assist in super-selective catheterization during CAIC for various types of cancer, including hepatic cancer (13,14). Thus, the purpose of this study was to evaluate the efficacy and safety of CACTA-guided super-selective CAIC in locally advanced GC patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-304/rc).
Methods
Patients and study design
In this retrospective study, patients with locally advanced GC were identified from the Second Affiliated Hospital of Zhejiang University School of Medicine between May 2018 and December 2018. Eligible patients were those aged 18 years or above, had histologically confirmed GC, and received three cycles of preoperative CAIC followed by surgery. Details regarding patient demographics, preoperative chemotherapy, radiographic re-evaluation, operation, pathology reports, adverse effects, and postoperative outcomes were collected via chart review of the patients’ medical records. The patients were then prospectively followed up for recurrence or progression of the disease at 3-month intervals for at least 1 year after surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the independent Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine (No.2021-1051). Written informed consent was obtained from each patient.
Preoperative staging and treatments
Preoperative staging was evaluated with contrast-enhanced CT according to the 8th edition of the Tumor Node Metastasis (TNM) classification (15). All patients received oral S-1 (80 mg/m2 twice daily for 14 days) plus CAIC with oxaliplatin (100 mg for 5 hours on day one) for three cycles, with each cycle lasting for 3 weeks. A modified fluorouracil, leucovorin and oxaliplatin (FOLFOX6) regimen was initially administrated due to the obstruction symptoms, and was replaced by S-1 plus Oxaliplatin (SOX) once the symptoms were alleviated. The modified FOLFOX6 consisted of a CAIC of oxaliplatin (100 mg/m2) on day 1, followed by an intravenous injection of leucovorin (400 mg/m2) on day 1, an intravenous injection of 5-fluorouracil (400 mg/m2), and then a continuous intravenous infusion of 5-fluorouracil (2,400 mg/m2) for 46 hours on day 1 every 3 weeks. After three cycles of NAC, the patients received total or subtotal gastrectomy with D2 lymphadenectomy.
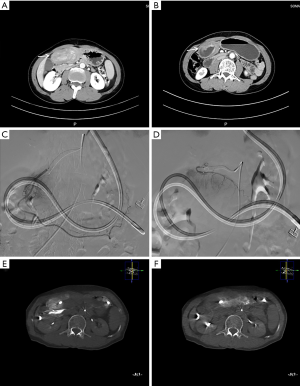
Intra-arterial oxaliplatin was administered via the femoral artery using the Seldinger’s technique. Briefly, a 2.4 F microcatheter (Merit Maestro microcatheter, Merit Medical, Utah, USA) was directed to a super-selective tumor feeding artery based on CACT in angiography. Oxaliplatin mixed with 250 mL of 5% glucose solution was then infused into the super-selective artery (50 mL/h). If more than one artery were involved in tumor feeding, the most important one was selected for intra-arterial infusion. A specific artery was selected accordingly in each cycle (Figure 1). All patients were re-evaluated for the feasibility of surgery via contrast-enhanced CT after three cycles of treatment. Distal gastrectomy with D2 lymphadenectomy was then performed.
Tumor response and adverse effects
Resected tumor specimens were examined for surgical margin status (R0, R1, and R2) and post-neoadjuvant pathological TNM (ypTNM) staging. The pathological response was graded according to the Becker regression criteria (16): (I) tumor regression grade (TRG)1a: complete pathological regression without any residual tumor cells; (II) TRG1b, subtotal regression with <10% residual tumor cells; (III) TRG2, partial regression with 10–50% residual tumor cells; and (IV) TRG3, minor or no regression with >50% residual tumor cells. The adverse events were collected from the patients’ medical records.
Follow-up
Every 3 months, all patients received face-to-face interviews at outpatient to know whether there were discomfort symptoms such as nausea, vomiting, abdominal distension, abdominal pain and obstruction. At the same time, they received abdominal enhanced CT and serum tumor markers to find out whether the tumor had recurrence and metastasis. The postoperative follow-up was requested for more than 1 year in all patients.
Results
Baseline patient characteristics
From May 2018 to December 2018, a total of four patients were included in this study, all of whom completed the 1-year postoperative follow-up. The baseline demographics and clinical characteristics of the patients are summarized in Table 1. All patients were diagnosed with stage IIIA/IIIB gastric adenocarcinoma with obstruction that required jejunal feeding.
Table 1
| Variables | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age, years | 57 | 59 | 65 | 71 |
| Sex | Female | Female | Male | Male |
| Tumor location | Gastric antrum | Gastric antrum | Gastric angle | Gastric antrum |
| Tumor invasion | cT4a | cT4a | cT4a | cT4a |
| LNM | cN3a | cN1 | cN1 | cN2 |
| cTNM staging | IIIB | IIIA | IIIA | IIIA |
| Obstruction | Yes | Yes | Yes | Yes |
LNM, lymph node metastasis.
Clinical and pathological findings
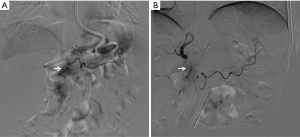
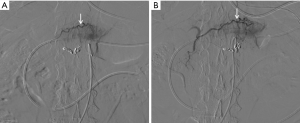
The patients’ obstructions were significantly alleviated. A liquid diet was possible 3–5 days after the first cycle of treatment. All patients resumed oral intake after three cycles of treatment. CT scan showed shrinkage in tumor volume and enlarged lymph nodes, as well as marked edema of the gastric wall adjacent to the tumor area (Figures 1,2). Also, compensatory enlargement of the non-target tumor feeding arteries was observed, along with atrophy of the target artery and decreased tumor staining on CAIC (Figures 3,4).
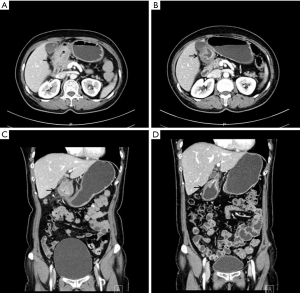
The surgical and pathological findings of the patients are shown in Table 2. R0 resection was achieved in all patients. Histopathological assessment showed tumor downstaging in three patients and upstaging in one patient. As for the pathological response, two patients achieved a complete (TRG1a) or subtotal (TRG1b) response, while the other two patients achieved a partial response (TRG2).
Table 2
| Variables | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Surgical margin | R0 | R0 | R0 | R0 |
| Tumor invasion | T0 | T4a | T4a | T4a |
| LNM | N0 | N0 | N0 | N2 |
| Distant metastasis | M0 | M0 | M0 | M1 |
| ypTNM stage | 0 | II | II | IV |
| TRG | TRG1a | TRG1b | TRG2 | TRG2 |
LNM, lymph node metastasis; TRG, tumor regression grade; ypTNM, post-neoadjuvant pathological Tumor Node Metastasis.
Meanwhile, no adverse events or perioperative complications were observed during or after treatment. All patients were alive without tumor recurrence or progression after the 1-year postoperative follow-up.
To sum up, the obstructive symptoms of all patients were relieved, tumor downstaging was evaluated by CT scan (4/4) and histopathology (3/4), conversion rate of R0 resection was 100%. The pathological response in all patient was 100%, including a complete/subtotal regression rate of 50%. There was no recurrence or metastasis after 1-year follow-up.
Discussion
Local intra-arterial administration of chemotherapeutic agents has been reported to produce 10-fold higher serum concentrations than systemic intravenous chemotherapy (2). The intra-arterial route is associated with a higher overall chemotherapy response rate than that of the systemic intravenous route. In our study, the tumor feeding artery of the included patients was defined using the CACTA technique. A microcatheter was then used to accurately deliver the chemotherapeutic drugs to the targeted arteries, so as to keep high local concentrations. Furthermore, the microcatheter was retained for a long time period (5 hours) to steadily perfuse chemotherapeutic drugs that can continuously act on tumor cells, while at the same time reducing the discomfort and incompliance of patients encountered during the CAIC.
The contrast-enhanced CT scan showed varying degrees of tumor volume reduction, which is a potential independent prognostic factor for advanced GC patients who had received NAC (17). Also, as a significant determinant of prognosis (18), tumor downstaging was also observed in three cases after CAIC. Tumor upstaging was noted in one case, since a nodule on the abdominal wall was not observed on CT scan, although the tumor and lymph node classification remained unchanged. TRG after preoperative chemotherapy has been established as an important prognostic factor in patients with advanced GC for tumor downstaging, recurrence, and patient survival (5). In this study, the TRG after CAIC were TRG1a (n=1), TRG1b (n=1), and TRG2 (n=2), with a complete/subtotal regression rate of 50% and an overall regression rate of 100%.
A higher overall pathological response rate [91.3% (42/46); 100% (78/78)] was observed in previous studies of advanced GC patients who received combined intravenous and intra-arterial neoadjuvant chemotherapies with FLEP (5-FU, leucovorin, etoposide, and cisplatin) or FLEEOX (5-FU, leucovorin, etoposide, oxaliplatin, and epirubicin) (19,20). However, a comparatively lower response rate was also noted in patients who received RAIC with EOX (56.1%, 46/82; epirubicin, oxaliplatin, and capecitabine) or EOF (42.4%, 72/170; epirubicin, oxaliplatin, fluorouracil, and calcium folinate) regimens (11,21). The potential reasons for this may be partially explained by the different regimens and methods of RAIC, as well as the criteria and cutoff levels used for pathologic response [Becker criteria, Mandard criteria, Japanese Classification of Gastric Carcinoma (JCGC) histological criteria]. Notably, the complete or subtotal pathologic response rates were only 11.54% and 30.43%, respectively, in the above-mentioned two studies of combined intravenous and intra-arterial neoadjuvant chemotherapies. The data were well within the range reported in patients treated with preoperative systemic NAC (19,20). In addition to the comparatively lower complete/subtotal pathologic response rates, the combined intravenous and intra-arterial procedures are complicated, which may limit their practical use as well as patient compliance. Based on what has been discussed above, super-selective CAIC showed promising efficacy in the neoadjuvant treatment of locally advanced GC patients.
Moreover, no obvious drug-related adverse events or perioperative complications were observed in multiple processes of CAIC in this study. This unexpected performance may be partly attributable to the lower dosage of the chemotherapy agents (approximately 1/2–2/3 of the recommended dose for systematic NAC) administrated locally into the tumor sites. Additionally, the CACTA-based micrometer system used for the super-selection of specific feeding arteries for long-term continuous infusion chemotherapy. These measures can not only ensure local drug concentration for tumor eradication, but also minimize the effect on the normal arteries. Taken together, our study showed good tolerance of CAIC in neoadjuvant treatment of locally advanced GC. The super-selective CAIC may serve as a promising alternative to systemic NAC in locally advanced GC patients.
Obstruction is commonly observed in advanced GC. Patients with obstruction often present with deeper cancer invasion and more lymph node metastases. Also, the survival rates of these patients are significantly lower compared with those without obstruction (12). The definitive role of chemotherapy as an initial treatment for relieving obstruction remains unclear (22). In this study, all four GC patients had obstruction that required jejunal feeding at baseline. The clinical symptoms were evidently improved after three treatment cycles, and oral intake was resumed in all patients. These results suggested that CAIC may be a potential alternative treatment option for GC patients with obstruction.
This study also has some limitations that should be noted. Firstly, only a few cases were included in this preliminary study. Secondly, radiologic response was not evaluable due to the marked edema of the gastric wall adjacent to the tumor area, which posed a major challenge in assessing the tumor response after treatment. Despite this, contrast-enhanced CT scanning showed shrinkage in tumor size and lymph nodes. Meanwhile, compensatory enlargement of the non-target tumor feeding arteries was also observed, along with atrophy of the target arteries and a decrease in tumor dyeing on CAIC (Figures 3,4). These results not only corroborated the report of Kruk-Bachonko et al. that enhancement of the GC lesion would weaken if chemotherapy was effective (1), but also acted as evidence for the effectiveness of neoadjuvant CAIC. Unfortunately, only 1-year postoperative follow-up was available, and thus, the long-term effects require further evaluation.
This preliminary pilot study showed promising efficacy and good tolerance of CAIC in NAC treatment of locally advanced GC. Future studies with a larger sample sizes and long-term outcomes are needed for a final conclusion.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-304/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-304/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-304/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kruk-Bachonko J, Krupski W, Czechowski M, et al. Perfusion CT - A novel quantitative and qualitative imaging biomarker in gastric cancer. Eur J Radiol 2017;95:399-408. [Crossref] [PubMed]
- Ji X, Yang Q, Qin H, et al. Tumor blood supply may predict neoadjuvant chemotherapy response and survival in patients with gastric cancer. J Int Med Res 2019;47:2524-32. [Crossref] [PubMed]
- Ahn HS, Jeong SH, Son YG, et al. Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. Br J Surg 2014;101:1560-5. [Crossref] [PubMed]
- Katayama H, Tsuburaya A, Mizusawa J, et al. An integrated analysis of two phase II trials (JCOG0001 and JCOG0405) of preoperative chemotherapy followed by D3 gastrectomy for gastric cancer with extensive lymph node metastasis. Gastric Cancer 2019;22:1301-7. [Crossref] [PubMed]
- Derieux S, Svrcek M, Manela S, et al. Evaluation of the prognostic impact of pathologic response to preoperative chemotherapy using Mandard's Tumor Regression Grade (TRG) in gastric adenocarcinoma. Dig Liver Dis 2020;52:107-14. [Crossref] [PubMed]
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697-708. [Crossref] [PubMed]
- Sah BK, Zhang B, Zhang H, et al. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun 2020;11:6093. [Crossref] [PubMed]
- Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: A meta-analysis of 17 published studies. Eur J Surg Oncol 2017;43:1607-16. [Crossref] [PubMed]
- Achilli P, De Martini P, Ceresoli M, et al. Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study. J Gastrointest Oncol 2017;8:1018-25. [Crossref] [PubMed]
- Aigner KR, Stephens FO. Induction chemotherapy: systemic and locoregional: Springer; 2016.
- Wu Z, Zhu W, Cao Q, et al. Application of regional arterial infusion chemotherapy in short-term neoadjuvant chemotherapy for advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:1092-5. [PubMed]
- Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 2018;69:60-9. [Crossref] [PubMed]
- Kim AY, Miller A. Evaluation of Surefire's precision direct-to-tumor embolization device to augment therapeutic response to intra-arterial, liver-directed therapies for patients with primary and secondary liver cancers. Expert Rev Med Devices 2016;13:435-43. [Crossref] [PubMed]
- Miyayama S, Yamashiro M, Hattori Y, et al. Usefulness of C-arm CT during superselective infusion chemotherapy for advanced head and neck carcinoma. J Med Imaging Radiat Oncol 2011;55:368-72. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. New York (NK): Springer; 2017.
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- Tang X, He Q, Qu H, et al. Post-therapy pathologic tumor volume predicts survival in gastric cancer patients who underwent neoadjuvant chemotherapy and gastrectomy. BMC Cancer 2019;19:797. [Crossref] [PubMed]
- Coimbra FJF, de Jesus VHF, Ribeiro HSC, et al. Impact of ypT, ypN, and Adjuvant Therapy on Survival in Gastric Cancer Patients Treated with Perioperative Chemotherapy and Radical Surgery. Ann Surg Oncol 2019;26:3618-26. [Crossref] [PubMed]
- Li GL, Liu K, Bao Y, et al. Retrospective analysis of 56 patients with advanced gastric cancer treated with combination of intravenous and intra-arterial intensified neoadjuvant chemotherapy. Chin Med J (Engl) 2012;125:780-5. [PubMed]
- He Q, Li Y, Ma L, et al. Application of FLEEOX Preoperative Chemotherapy via Intra-arterial and Intravenous Administration in Treatment of Unresectable Locally Advanced Gastric Cancer. J Gastrointest Surg 2016;20:1421-7. [Crossref] [PubMed]
- Wu ZF, Cao QH, Wu XY, et al. Regional Arterial Infusion Chemotherapy improves the Pathological Response rate for advanced gastric cancer with Short-term Neoadjuvant Chemotherapy. Sci Rep 2015;5:17516. [Crossref] [PubMed]
- Jiao X, Zhou Y. Investigation of the potential role of preoperative chemotherapy in treatment for gastric cancer with outlet obstruction. Mol Clin Oncol 2015;3:1177-83. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


