
Jejunojejunal intussusception with chronic bleeding caused by gastrointestinal stromal tumor: a case report and literature review
Introduction
Intussusception rarely occurs in adults, with an annual incidence of approximately 2–3 per million people (1,2). Unlike pediatric intussusception cases, 90% of which are idiopathic, most adult intussusception cases are secondary to benign or malignant neoplasms such as gastrointestinal stromal tumors (GISTs) (1). However, GISTs comprise less than 0.2% of all gastrointestinal tumors and only 0.04% of small intestinal tumors (3). Gastrointestinal bleeding is a frequent symptom of GISTs (4), while only 5% of all gastrointestinal bleeding occurs in the small bowel (5). These small bowel lesions are usually difficult to find and subject to late diagnosis, leading to treatment delays. Herein, we present a rare case of adult small bowel intussusception with chronic bleeding caused by a GIST. We emphasize the role of contrast-enhanced computed tomography (CT) and enteroscopy, which allowed presurgical visualization of the lesion, and the good prognosis in the patient with high-risk GIST after surgery and targeted therapy. We present the following case in accordance with the CARE reporting checklist (6) (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-301/rc). In addition, we performed a literature search and review of case reports of small bowel intussusception caused by GISTs.
Case presentation
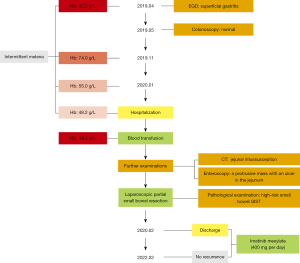
A 63-year-old Chinese woman presented to the Department of General Surgery, Strategic Support Force Medical Center in January 2020 with intermittent melena and anemia without an obvious cause for 9 months. In early April 2019, the patient had begun to pass recurring tarry stools without mucus or pus, unaccompanied by abdominal pain, jaundice, fever, hematemesis, nausea, or vomiting. Tests at a district hospital returned a hemoglobin level (Hb) of 80.0 g/L and a mean corpuscular hemoglobin (MCH) level of 24.2 pg. Her fecal occult blood test returned positive. Esophagogastroduodenoscopy (EGD) and colonoscopy showed no gastrointestinal pathology to account for the bleeding. After pharmacological treatment, the melena improved in the short-term but later reappeared. In January 2020, the patient’s blood routine was retested due to increasing fatigue and dyspnea. Her Hb at this time was 55.0 g/L, and her MCH was 17.6 pg. The patient had a 20-year history of hypertension and had never undergone previous abdominal surgery. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
She was admitted to Strategic Support Force Medical Center for further diagnosis and treatment. Her pulse and blood pressure were normal, but she had a deficient nutritional status with pale skin and mucous membranes. A physical examination revealed mild tenderness around the umbilicus with no abdominal muscle tension, rebound pain, or mass. Bowel sounds were slightly active, about 6–7 times per minute. Blood analysis showed an Hb of 48.2 g/L, an MCH of 17.6 pg, and normal platelet levels. Blood biochemistry and coagulation indicators were normal. Abdominal CT revealed a jejunal intussusception (Figure 1).
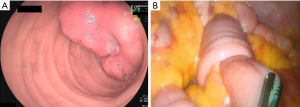
Continued blood transfusion restored the patient’s Hb to 94.0 g/L and provided safe conditions for further invasive examination and treatment. A small bowel enteroscopy revealed a protrusive mass of about 3.5 cm × 4.0 cm in the small intestine, 50 cm from the pylorus, with an ulcer at the top covered with white moss (Figure 2A). After identification of the bleeding lesion, the patient underwent an immediate laparoscopic partial small bowel resection. Intraoperative exploration revealed a palpable small intestinal mass about 50 cm from the Treitz ligament. The proximal section of the small intestine with the mass was inserted into the distal bowel, which had formed an intussusception (Figure 2B).
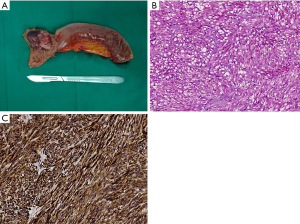
Gross examination of the surgical specimen revealed a greyish-white soft tissue mass, measuring about 3 cm × 4 cm, with a brown area of hemorrhage and a mucosal ulcer on the tumor surface (Figure 3A). Histopathological examination [hematoxylin and eosin (H&E) stain, ×200] showed a small intestinal spindle cell neoplasm from the mucosa to the deep muscularis (Figure 3B). There were more than 10 mitoses per 50 high-power fields (HPF). The surgical margin was negative, and there was no obvious intravascular tumor thrombus or nerve invasion. Immunohistochemical (IHC) staining showed that the tumor was diffusely positive for CD117 (KIT), DOG1, and CD34 but negative for smooth muscle actin, desmin, and S100 (Figure 3C). Accordingly, the diagnosis of a high-risk small bowel GIST was made.
The patient recovered without complications and was discharged on the 17th postoperative day. The patient started taking imatinib besylate (400 mg per day) 1 month after surgery due to the high risk of recurrence. It was suggested that if no severe adverse reaction occurred, she should continue to use the drug for at least 3 years. Currently, we are conducting regular follow up by phone. At the time of writing, the patient is still taking imatinib regularly and has remained relapse-free for 24 months post-discharge. No adverse reactions to the targeted drug have been observed so far. She has expressed great satisfaction with the results of her surgery and targeted therapy. A timeline is shown in Figure 4.
Discussion
Intussusception is the telescoping or invagination of the proximal part of the gastrointestinal tract (intussusceptum) into an adjacent section (intussuscipiens). Adult intussusception is uncommon, accounting for 5% of all intussusception cases (2). The main etiologies of adult intussusception are various underlying intestinal lesions, such as inflammatory bowel disease, postoperative adhesions, Meckel’s diverticulum, and benign and malignant tumors (7). Adult intussusception is an indication for surgical resection. Intussusception caused by GISTs is rare because most GISTs are sporadic and tend to grow in an extraluminal fashion (8). The GISTs probably originate from the interstitial cells of Cajal or their cellular progenitors, which are responsible for regulating peristalsis and serve as pacemakers in the gastrointestinal tract (9). It has been reported that small bowel GISTs present less frequently than gastric GISTs, but carry a higher malignancy (10).
The GISTs are the most common primary mesenchymal tumors of the gastrointestinal tract and possess a wide range of malignant potential (10). They occur most frequently in the stomach (55.6%) and small intestine (31.8%) (11). Most GISTs seem to be sporadic, and risk factors are poorly understood. However, some GISTs might be associated with specific tumor syndromes, such as familial GIST, Carney’s triad, Carney-Stratakis syndrome, or type 1 neurofibromatosis (NF1) (9). The primary symptoms are usually nonspecific, including abdominal pain, gastrointestinal bleeding, and anemia. Other symptoms may include dyspepsia, nausea or vomiting, constipation or diarrhea, frequent urination, and fatigue (9). Sometimes, GISTs irritate and alter normal peristaltic activity, triggering an intraluminal invagination and finally causing an intussusception (12). Endoscopy is recommended as the primary diagnostic modality for symptoms such as hematochezia or melena. When a source is not found after a routine endoscopy, further diagnostic workup includes repeat testing and evaluation of the small bowel, often using enteroscopy and wireless capsule endoscopy. In addition, endoscopic ultrasound, contrast-enhanced CT, or magnetic resonance imaging (MRI), and interventional angiography can help to locate the bleeding point and primary lesion. In the abovementioned case, the primary symptoms of the patient were anemia and melena. As both the EGD and colonoscopy were negative, the possibility of a small bowel bleeding lesion was considered. Abdominal contrast-enhanced CT and enteroscopy were subsequently performed, and small bowel intussusception and a bleeding lesion were confirmed.
Surgery is the primary therapeutic option for a GIST, with the goal being complete resection for non-metastatic tumors. Lymph node metastases are rare (prevalence is about 1%) and routine removal of the lymph nodes is typically unnecessary (4). Age, comorbidity, performance status, tumor location, size, and resection margin should be considered when selecting the type of surgical resection for GISTs. A small bowel GIST is usually resected with a bowel segment.
The pathologic diagnosis of a GIST mainly depends on IHC characteristics. More than 95% of GISTs are positive for CD117 (KIT) and/or DOG1 (13). If the tumor cells are negative for CD117 and DOG1 in highly suspicious cases, mutational analysis for the KIT or platelet-derived growth factor receptor alpha (PDGFRA) gain-of-function mutations may help to confirm the diagnosis of a GIST. Moreover, mutation detection has important significance in guiding targeted therapy and prognostic assessment for patients with this type of tumor. The European Society for Medical Oncology, European Reference Network for Rare Adult Solid Cancers, and European Reference Network for Genetic Tumor Risk Syndromes Clinical Practice Guidelines (ESMO-EURACAN-GENTURIS CPGs) recommend that mutational analysis be considered as a routine examination in the diagnostic workup of all GISTs (4). However, 10–15% of GISTs do not have a detectable KIT or PDGFRA mutation. These are called wild-type GISTs and include GISTs in NF1, Carney-Stratakis syndrome, Carney’s triad, BRAF mutation, succinate dehydrogenase (SDH) subunit mutations (SDHA, SDHB, SDHC, SDHD), and RAS-family mutations (HRAS, NRAS, KRAS) (9). The immunoreactivity of SDHB can be used to identify SDH-deficient GISTs (14). In GISTs, the mitotic index has more important prognostic significance than Ki-67 analysis. The tumor in our case exhibited diffuse CD117 and DOG1 positivity. The mitotic index indicated that the patient was at high risk of early recurrence. Imatinib mesylate, an oral tyrosine kinase inhibitor, has been taken by the patient for 24 months to date to prevent recurrence after surgery, and she remains in good health without relapse at the time of writing.
We searched PubMed with the search terms “intussusception”, “small bowel” and “gastrointestinal stromal tumor” to identify relevant studies. Only 20 cases of intussusception caused by small bowel GISTs have been reported (Table 1). Of the 21 patients (including our case), 12 were female, and 9 were male. The age of the patients ranged from 6 to 91 years, with a median age of 55 years. The median size of the maximum tumor diameter was 4 cm (1.5–15 cm). A total of 13 patients (62%) presented with melena or hematochezia on admission. The data showed that 95% (n=20) were CD117-positive, and 5% (n=1) were CD117-negative. All patients underwent curative surgery, and 6 patients were treated with imatinib after surgery. A 79-year-old woman with an ileoileal intussusception secondary to a terminal ileum GIST underwent resection of the neoplastic segment and a wide mesenteric lymphadenectomy (16). The pathology report showed all identified mesenteric lymph nodes were negative for malignancy. Wall et al. (19) reported the case of a 36-year-old woman with a 15-cm GIST in the second part of the duodenum who underwent pylorus-preserving pancreaticoduodenectomy due to the large size of the tumor and inseparable adhesion to the head of the pancreas. A 91-year-old Japanese woman with a GIST in the third portion of the duodenum received a segmental duodenectomy without reduction of the intussusception but did not undergo a pancreaticoduodenectomy due to her age and performance status (30). All other patients (including our patient) underwent a curative partial small bowel resection. The median follow-up period of all patients was 7 months (2–24 months), and no tumor recurrences were reported.
Table 1
| Author | Country/ region |
Year | Patient age (years) | Gender | Presentation | Surgical approach | Tumor size (cm) | c-Kit/CD117, mitotic index (mitosis/50 HPF) | Adjuvant therapy | Follow-up (months), recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Hoshino et al. (15) | Japan | 2000 | 42 | F | Anemia, massive melena, abdominal pain | Partial small bowel resection | 3 | Positive, 5–8 | No | 11, no recurrence |
| Vasiliadis et al. (16) | Greece | 2008 | 79 | F | Lower abdominal colicky pain, abdominal distension, nausea, vomiting, constipation | Partial small bowel resection, wide mesenteric lymphadenectomy | 2.2 | Positive, 7–8 | No | 7, no recurrence |
| Menendez-Sanchez et al. (17) | Spain | 2009 | 55 | F | Abdominal pain, rectal bleeding | Partial small bowel resection | 5 | Positive, NR | No | NR, no recurrence |
| Theodoropoulos et al. (8) | Greece | 2009 | 45 | M | Abdominal pain, vomiting, obstipation, abdominal distention | Partial small bowel resection | 6 | Positive, NR | NR | 14, no recurrence |
| Rabbani et al. (18) | Morocco | 2010 | 59 | F | Abdominal distension and pain, obstipation, vomiting | Partial small bowel resection | NR | Positive, NR | NR | NR |
| Wall et al. (19) | UK | 2010 | 36 | F | Collapse, melena, | Pancreaticoduodenectomy | 15 | Positive, NR | No | NR, no recurrence |
| Ku et al. (20) | Taiwan | 2011 | 39 | F | Melena, anemia | Partial small bowel resection | 1.5 | Positive, NR | NR | NR |
| Dhull et al. (21) | India | 2011 | 38 | M | Abdominal pain | Partial small bowel resection | 15 | Positive, 6 | Yes | 6, no recurrence |
| Sam et al. (22) | Canada | 2011 | 50 | M | Intermittent melena, mild cramping abdominal pain, anemia | Partial small bowel resection | 2.5 | Positive, 0 | No | 6, no recurrence |
| Gupta et al. (23) | India | 2011 | 59 | M | Abdominal pain, distension, vomiting, constipation | Partial small bowel resection | NR | Positive, NR | Yes | NR |
| Bhanvadia et al. (24) | India | 2012 | 75 | F | Abdominal pain, vomiting, abdominal distention, constipation | Partial small bowel resection | 4.5 | Negative, >5 | NR | NR |
| Gunaydin et al. (25) | Turkey | 2012 | 6 | F | Abdominal pain, vomiting | Partial small bowel resection | 2.5 | Positive, 2–3 | No | 14, no recurrence |
| Basu et al. (26) | India | 2014 | 46 | F | Abdominal pain, abdominal distension, anorexia, vomiting, constipation | Partial small bowel resection | 4 | Positive, 6 | Yes | 24, no recurrence |
| Chen et al. (27) | China | 2014 | 34 | F | Abdominal pain, melena | Partial small bowel resection | 2.5 | Positive, NR | NR | NR |
| Sadeghi et al. (5) | UK | 2015 | 68 | M | Melena, anemia, abdominal pain and distension, vomiting and constipation | Partial small bowel resection | 4 | Positive, 0-1 | Yes | NR, no recurrence |
| Sankey et al. (28) | UK | 2015 | 70 | M | Abdominal pain, nausea, vomiting, constipation, melena, anemia | Partial small bowel resection | 4 | Positive, NR | Yes | 3, no recurrence |
| Rahimi et al. (29) | USA | 2016 | 77 | M | Abdominal discomfort, vomiting, melena, syncope, anemia | Laparoscopic small bowel resection | 3 | Positive, 1-2 | NR | NR |
| Fujimoto et al. (30) | Japan | 2019 | 91 | F | Vomiting, anorexia, anemia, hypoproteinemia | Palliative segmental duodenectomy | 4 | Positive, 20 | NR | 6, no recurrence |
| Kim et al. (3) | South Korea | 2019 | 51 | M | Massive hematochezia, hypotension, abdominal pain, anemia | Partial small bowel resection | 3.5 | Positive, <5 | NR | 2, no recurrence |
| Burch et al. (31) | India | 2021 | 76 | M | Melena | Laparoscopic small bowel resection | NR | Positive, NR | NR | NR |
| Our case | China | 2022 | 63 | F | Melena, anemia | Partial small bowel resection | 4 | Positive, >10 | Yes | 24, no recurrence |
GIST, gastrointestinal stromal tumor; F, female; M, male; NR, not reported; HPF, high-power field; USA, United States of America; UK, United Kingdom.
The strengths of our case were listed as follows. Firstly, among the various gastrointestinal bleeding pathologies, small intestinal bleeding is uncommon, and adult jejunojejunal intussusception with gastrointestinal bleeding caused by a small intestinal GIST is extremely rare. Secondly, timely diagnoses of small bowel intussusception and bleeding tumor were made after contrast-enhanced CT and enteroscopy. After surgical resection and adjuvant targeted therapy, the high-risk GIST patient got a favorable prognosis. Among 20 cases of intussusception caused by small bowel GISTs reported, only one had a disease-free survival time of 24 months (22), and our case had a higher mitotic index (>10 vs. 6 mitosis/50 HPF). There are several limitations of this study: this is a case report, so the findings cannot be generalized. Besides, genetic analysis was not performed to further clarify the specific classification. More cases should be collected, and deeper studies should be done.
In summary, adult jejunojejunal intussusception with chronic gastrointestinal bleeding caused by a small intestinal GIST is very rare. If both EGD and colonoscopy fail to find the hemorrhage foci in the gastrointestinal tract, contrast-enhanced CT and enteroscopy can be performed to identify the small bowel lesion. Comprehensive surgical resection-centered treatment is the standard treatment for primary localized GISTs in patients at significant risk of relapse.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-301/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-301/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ssentongo P, Egan M, Arkorful TE, et al. Adult Intussusception due to Gastrointestinal Stromal Tumor: A Rare Case Report, Comprehensive Literature Review, and Diagnostic Challenges in Low-Resource Countries. Case Rep Surg 2018;2018:1395230. [Crossref] [PubMed]
- Tarchouli M, Ait AA. Adult Intussusception: An Uncommon Condition and Challenging Management. Visc Med 2021;37:120-7. [Crossref] [PubMed]
- Kim MS, Woo IT, Jo YM, et al. Life-threatening bleeding with intussusception due to gastrointestinal stromal tumor: a case report. Surg Case Rep 2019;5:154. [Crossref] [PubMed]
- Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022;33:20-33. [Crossref] [PubMed]
- Sadeghi P, Lanzon-Miller S. A jejunal GIST presenting with obscure gastrointestinal bleeding and small bowel obstruction secondary to intussusception. BMJ Case Rep 2015;2015:bcr2014207650. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Panzera F, Di Venere B, Rizzi M, et al. Bowel intussusception in adult: Prevalence, diagnostic tools and therapy. World J Methodol 2021;11:81-7. [Crossref] [PubMed]
- Theodoropoulos GE, Linardoutsos D, Tsamis D, et al. Gastrointestinal stromal tumor causing small bowelintussusception in a patient with Crohn’s disease. World J Gastroenterol 2009;15:5224-7. [Crossref] [PubMed]
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. [Crossref] [PubMed]
- Yuan J, Kihara T, Kimura N, et al. Differential Expression of CADM1 in Gastrointestinal Stromal Tumors of Different Sites and with Different Gene Abnormalities. Pathol Oncol Res 2021;27:602008. [Crossref] [PubMed]
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. [Crossref] [PubMed]
- Atalaia-Martins C, Barbeiro S, Marcos P, et al. Ileo-ileal intussusception as an unusual cause of obscure overt gastrointestinal bleeding. Endoscopy 2016;48:E386-7. [Crossref] [PubMed]
- Novelli M, Rossi S, Rodriguez-Justo M, et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 2010;57:259-70. [Crossref] [PubMed]
- Mei L, Smith SC, Faber AC, et al. Gastrointestinal Stromal Tumors: The GIST of Precision Medicine. Trends Cancer 2018;4:74-91. [Crossref] [PubMed]
- Hoshino N, Murata T, Oka K, et al. Gastrointestinal stromal tumors of the small intestine that expressed c-kit protein. Intern Med 2000;39:914-9. [Crossref] [PubMed]
- Vasiliadis K, Kogopoulos E, Katsamakas M, et al. Ileoileal intussusception induced by a gastrointestinal stromal tumor. World J Surg Oncol 2008;6:133. [Crossref] [PubMed]
- Menéndez-Sánchez P, Villarejo-Campos P, Gambí-Pisonero D, et al. Gastrointestinal bleeding and intussusception due to gastrointestinal stromal tumor (GIST). Cir Cir 2009;77:451-3. [PubMed]
- Rabbani K, Narjis Y, Finech B, et al. Unusual malignant cause of adult intussusception: Stromal tumor of the small bowel. J Emerg Trauma Shock 2010;3:306. [Crossref] [PubMed]
- Wall ML, Ghallab MA, Farmer M, et al. Gastrointestinal stromal tumour presenting with duodenal-jejunal intussusception: a case report. Ann R Coll Surg Engl 2010;92:W32-4. [Crossref] [PubMed]
- Ku MC, Tsai CM, Tyan YS. Gastrointestinal bleeding in a type 1 neurofibromatosis patient. Gastroenterology 2011;141:437-8, 782. [Crossref] [PubMed]
- Dhull AK, Kaushal V, Dhankhar R, et al. The inside mystery of jejunal gastrointestinal stromal tumor: a rare case report and review of the literature. Case Rep Oncol Med 2011;2011:985242. [Crossref] [PubMed]
- Sam JJ, Mustard R, Kandel G, et al. Colonoscopy Leads to A Diagnosis of A Jejunal Gastrointestinal Stromal Tumour (GIST). Gastroenterology Res 2011;4:277-82. [Crossref] [PubMed]
- Gupta A, Gupta S, Tandon A, et al. Gastrointestinal stromal tumor causing ileo-ileal intussusception in an adult patient a rare presentation with review of literature. Pan Afr Med J 2011;8:29. [Crossref] [PubMed]
- Bhanvadia VM, Trivedi B, Sheikh SS, et al. CD117 (C-Kit)-Negative Jejunal Epithelioid Gastrointestinal Stromal Tumour (GIST) Presenting as Intussusception. J Gastrointest Cancer 2012;43:S97-100. [Crossref] [PubMed]
- Gunaydin M, Bıçakcı Ü, Bozkurter AT, et al. Gastrointestinal stromal tumor: a very rare cause of jejunoileal intussusception in a 6-year-old girl. J Pediatr Surg 2012;47:e15-8. [Crossref] [PubMed]
- Basu A, Dutta MK, De U, et al. Jejunojejunal intussusception caused by a jejunal gastrointestinal stromal tumour (GIST). Hell Cheirourgike 2014;86:37-41. [Crossref]
- Chen YT, Sun HL, Luo JH, et al. Interventional digital subtraction angiography for small bowel gastrointestinal stromal tumors with bleeding. World J Gastroenterol 2014;20:17955-61. [Crossref] [PubMed]
- Sankey RE, Maatouk M, Mahmood A, et al. Case Report: Jejunal gastrointestinal stromal tumour, a rare tumour, with a challenging diagnosis and a successful treatment. J Surg Case Rep 2015;2015:rjv050. [Crossref] [PubMed]
- Rahimi E, Guha S, Chughtai O, et al. Role of enteroscopy in the diagnosis and management of adult small-bowel intussusception. Gastrointest Endosc 2016;84:863-4. [Crossref] [PubMed]
- Fujimoto G, Osada S. Duodenojejunal intussusception secondary to primary gastrointestinal stromal tumor: A case report. Int J Surg Case Rep 2019;64:15-9. [Crossref] [PubMed]
- Burch J, Ahmad I. Jejunal Gastrointestinal Stromal Tumor as a Source of Small Bowel Bleeding: A Case Report. Perm J 2021;25:20.257.
(English Language Editor: J. Jones)



