
Prognosis of patients with esophageal squamous cell carcinoma undergoing surgery versus no surgery after neoadjuvant chemoradiotherapy: a retrospective cohort study
Introduction
Esophageal cancer (EC) is the sixth most common cancer and the fourth leading cause of cancer death in China (1). Cases originating in China account for about 53% of new cases of EC worldwide (2). At present, the clinical treatment of EC is mainly surgical resection, radiotherapy and chemotherapy. EC can be divided into two subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). EAC is dominant in the western population, but in China, more than 90% of EC is ESCC (2). ESCC has the characteristics of easy metastasis and recurrence. It’s reported that 60–70% of Chinese patients are diagnosed with locally advanced EC that cannot be cured by surgery alone (1). Recent studies have confirmed that Neo-CRT combined with surgery is the standard treatment for locally advanced ESCC (3,4). It has been reported that the pathological complete response (pCR) rate may be up to 49% after ESCC (5). The relatively high rate of pCR, combined with the high invasive and risk of esophageal surgery, make some clinicians prefer a wait-and-see approach with close follow-up. Therefore, for patients with ESCC after Neo-CRT, whether to continue surgery has become a clinical problem. We believe that it is necessary to study the prognosis of patients undergoing surgery and non-operation after Neo-CRT. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-296/rc).
Methods
Study design and patient population
This was a retrospective cohort study. We retrospectively studied patients with locally advanced ESCC who were treated in Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University from June 2007 to December 2014. Strict control of inclusion and exclusion criteria was required due to possible research selection bias. The inclusion criteria were as follows: Clinical stage T1-4N1M0/T4N0M0 (second stage B or third stage) according to American Joint Committee on Cancer (AJCC) cancer staging manual 8th edition; age 18–70 years; hematology, kidney, and liver function were normal; and the Karnofsky performance score of ≥90. We excluded patients with a history of other malignant tumors; those who were not suitable for surgery due to comorbidities. We collected data on 451 patients with locally advanced ESCC. A total of 227 patients who underwent surgery alone were excluded. In addition, 1 patient who received neither Neo-CRT nor surgery was excluded from the study. A total of 223 patients (189 males and 34 females) were enrolled (Figure 1). At the same time, 38 of these patients did not undergo surgery after Neo-CRT (non-operation group) and 185 patients underwent surgery after Neo-CRT (operation group).
The study was conducted in accordance with the Declaration Helsinki (as revised in 2013). This study was approved by Medical Ethics Committee of Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University (No. K20220423). Individual consent for this retrospective analysis was waived.
Neo-CRT
All cases were administered intravenous infusion of vinorelbine 25 mg/m2 on days 1 and 8, cisplatin 75 mg/m2 within 3 hours on day 1 or cisplatin 25 mg/m2 within 2 hours on days 1 to 4, every 3 weeks for 2 cycles. At the same time, radiation therapy was given at 2.0 Gy once, 5 times a week, with a total dose of 40.0 Gy. All cases received external irradiation using three-dimensional (3D) conformal radiotherapy.
Operation time
Patients in non-operation group did not undergo surgery for various reasons, and those in operation group underwent surgery 4–6 weeks after the end of radiotherapy and chemotherapy. Esophagectomy was performed on the right transthoracic (McKeown or Ivor Lewis), including thoraco and abdominal lymph node dissection.
Outcomes and follow-up
The primary outcome of this study was overall survival (OS), defined as the time from the date of enrollment to the date of death or last follow-up. Adverse reactions to Neo-CRT were assessed according to The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (https://ctep.cancer.gov). All patients were followed up regularly through outpatient appointments or telephone calls. Clinical follow-up is performed every 3 months for the first year and every 6 months thereafter until the patient dies or the deadline. Information on the first postoperative recurrence was used for this study. The data cutoff for the analysis presented here was 31 December, 2019.
Statistical analysis
The software SPSS 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. We collected the clinical characteristics, adverse reactions of Neo-CRT, recurrence and survival, and evaluated the prognosis of the two groups. The baseline characteristics of the patients were compared with Student’s t-test and Mann-Whitney U test. Clinical stages and adverse reactions were compared by chi-squared test. The OS was calculated by Kaplan-Meier method and compared by log-rank test. Cox proportional hazards model was used for univariate and multivariate analysis to evaluate the impact of different factors on survival, expressed as hazard ratios (HRs). Covariates included age, gender, tumor location, clinical T stage, N stage, surgery, and recurrence. All tests were set at 2-tailed P<0.05.
Results
Patient characteristics
A total of 223 patients with locally advanced ESCC treated in Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University from June 2007 to December 2014 were included. The detailed clinical features of 223 patients are shown in Table 1. In non-operation group, 32 patients (84.2%) were male, and in operation group, 157 patients (84.9%) were male. The median age of patients in the two groups was 58 and 53 years old [interquartile range (IQR), 41 to 70 and 31 to 70], respectively. The average body mass index (BMI) was 21.88 [standard deviation (SD) =1.64] and 22.24 (SD=3.06) kg/m2, respectively. Most patients were middle thoracic ESCC, with 28 cases in non-operation group (73.7%) and 129 cases in operation group (69.7%). There were 15 cases (39.5%) with clinic T3 and 19 cases (50.0%) with clinic T4 in non-operation group. Meanwhile there were 108 cases (58.4%) with T3 and 45 cases (24.3%) with T4 in non-operation group. Most patients had lymph node metastasis (27 vs. 163, 71.1% vs. 88.1% in non-operation group and operation group, respectively).
Table 1
| Characteristic | Non-operation group (n=38) | Operation group (n=185) | P value |
|---|---|---|---|
| Age (years) | 0.025 | ||
| Median | 58 | 53 | |
| IQR | 41–70 | 31–70 | |
| Gender | 0.919 | ||
| Male | 32 (84.2) | 157 (84.9) | |
| Female | 6 (15.8) | 28 (15.1) | |
| BMI (kg/m2) | 0.300 | ||
| Mean | 21.88 | 22.24 | |
| SD | 1.64 | 3.06 | |
| KPS | 0.170 | ||
| 90 | 37 (100.0) | 185 (100.0) | |
| 100 | 1 (2.6) | 0 (0.0) | |
| Tumor location | 0.343 | ||
| Proximal third | 6 (15.8) | 20 (10.8) | |
| Middle third | 28 (73.7) | 129 (69.7) | |
| Distal third | 4 (10.5) | 36 (19.5) | |
| Clinical T stage | 0.006 | ||
| T1–2 | 4 (10.5) | 32 (17.3) | |
| T3 | 15 (39.5) | 108 (58.4) | |
| T4 | 19 (50.0) | 45 (24.3) | |
| Clinical N stage | 0.007 | ||
| N0 | 11 (28.9) | 22 (11.9) | |
| N1 | 27 (71.1) | 163 (88.1) | |
| Clinical stage | 0.301 | ||
| IIB | 4 (10.5) | 32 (17.3) | |
| III | 34 (89.5) | 153 (82.7) |
Data are presented as No. (%). IQR, interquartile range; BMI, body mass index; SD, standard deviation; KPS, Karnofsky performance score.
Among 223 patients with Neo-CRT, 38 cases did not undergo surgery, with a non-operative rate of 17.0%. There were many reasons for not undergoing surgery, including 29 patients who refused surgery, 2 patients with disease progression, 2 patients with poor general condition who could not tolerate surgery, 1 patient with cerebral infarction, and 4 patients who died of pneumonia, esophageal bleeding (n=2), and a car accident, respectively (Table 2).
Table 2
| Discontinued surgery | n | Percentage (%) |
|---|---|---|
| Refused surgery | 29 | 76.3 |
| Disease progression | 2 | 5.3 |
| Could not tolerate surgery | 2 | 5.3 |
| Died before surgery | ||
| Esophageal hemorrhage | 2 | 5.3 |
| Pneumonia | 1 | 2.6 |
| Accident | 1 | 2.6 |
| Cerebral infarction | 1 | 2.6 |
Adverse reactions
In non-operation group, 18.4% (7/38) of patients received only 1 course of chemotherapy, while 11.4% (21/185) of patients in operation group received only 1 course of chemotherapy. There was no statistical difference between the two groups in the course of chemotherapy (P=0.353). The most common adverse reactions after Neo-CRT in both groups were leukopenia (84.2% and 80%, respectively), followed by neutropenia (81.6% and 72.4%, respectively), with no statistical difference (P=0.549 and P=0.242). The hemoglobin hypoplasia rate was 73.7% (28/38) in non-operation group and 53.0% (98/185) in operation group, and the difference was statistically significant (P=0.019). There were significant differences in infection rate (15.8% and 2.7%, P=0.003) and fatigue rate (2.6% and 18.9%, P=0.013), respectively, between the two groups, while there were no significant differences in other adverse reactions (Table 3). Leucopenia was the most common grade 3 or above adverse reaction in both groups (63.2% and 45.9%, respectively, P=0.090). In terms of the distribution of adverse reactions, there were differences in low hemoglobin, thrombocytopenia, fatigue, and infection between the two groups (Table 4).
Table 3
| Adverse reaction | Non-operation group (n=38) | Operation group (n=185) | P value |
|---|---|---|---|
| Low hemoglobin | 28 (73.7) | 98 (53.0) | 0.019 |
| Leukopenia | 32 (84.2) | 148 (80.0) | 0.549 |
| Neutropenia | 31 (81.6) | 134 (72.4) | 0.242 |
| Thrombocytopenia | 20 (52.6) | 69 (37.3) | 0.079 |
| AST or ALT increase | 5 (13.2) | 18 (9.7) | 0.734 |
| Anorexia | 20 (52.6) | 105 (56.8) | 0.641 |
| Vomiting | 24 (63.2) | 102 (55.1) | 0.462 |
| Diarrhea | 3 (7.9) | 12 (6.5) | 1.000 |
| Constipation | 3 (7.9) | 23 (12.4) | 0.606 |
| Mucositis | 3 (7.9) | 12 (6.5) | 1.000 |
| Radiation esophagitis | 15 (39.5) | 69 (37.3) | 0.801 |
| Radiodermatitis | 2 (5.3) | 13 (7.0) | 0.968 |
| Cough | 4 (10.5) | 18 (9.7) | 1.000 |
| Fatigue | 1 (2.6) | 35 (18.9) | 0.013 |
| Fever without infection | 1 (2.6) | 17 (9.2) | 0.305 |
| Infection | 6 (15.8) | 5 (2.7) | 0.003 |
Data are presented as No. (%). AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 4
| Adverse reaction | Non-operation group | Operation group | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Grade 0 | Grade 1–2 | Grade ≥3 | Grade 0 | Grade 1–2 | Grade ≥3 | |||
| Low hemoglobin | 10 (26.3) | 24 (73.2) | 4 (10.5) | 87 (47.0) | 93 (50.2) | 5 (2.7) | 0.007 | |
| Leukopenia | 6 (15.8) | 8 (21.1) | 24 (63.2) | 37 (20.0) | 63 (34.1) | 85 (45.9) | 0.090 | |
| Neutropenia | 7 (18.4) | 9 (23.7) | 22 (57.9) | 51 (26.0) | 54 (28.3) | 80 (43.2) | 0.099 | |
| Thrombocytopenia | 18 (47.4) | 13 (34.2) | 7 (18.4) | 116 (62.7) | 60 (32.4) | 9 (4.9) | 0.027 | |
| AST or ALT increase | 33 (86.8) | 5 (13.2) | 0 (0) | 167 (90.3) | 18 (9.7) | 0 (0) | 0.528 | |
| Anorexia | 18 (47.4) | 19 (50.0) | 1 (2.6) | 80 (43.2) | 101 (54.6) | 4 (2.2) | 0.675 | |
| Vomiting | 14 (36.8) | 22 (57.9) | 2 (5.3) | 83 (44.9) | 95 (51.4) | 7 (3.8) | 0.346 | |
| Diarrhea | 35 (92.1) | 3 (7.9) | 0 (0) | 173 (93.5) | 12 (6.5) | 0 (0) | 0.753 | |
| Constipation | 35 (92.1) | 3 (7.9) | 0 (0) | 162 (87.6) | 23 (12.4) | 0 (0) | 0.428 | |
| Mucositis | 35 (92.1) | 3 (7.9) | 0 (0) | 173 (93.5) | 10 (5.4) | 2 (1.1) | 0.767 | |
| Radiation esophagitis | 23 (60.5) | 14 (36.8) | 1 (2.6) | 116 (62.7) | 64 (34.6) | 5 (2.7) | 0.810 | |
| Radiodermatitis | 36 (94.7) | 2 (5.3) | 0 (0) | 172 (93.0) | 13 (7.0) | 0 (0) | 0.693 | |
| Cough | 34 (89.5) | 3 (7.9) | 1 (2.6) | 167 (90.3) | 18 (9.7) | 0 (0) | 0.843 | |
| Fatigue | 37 (97.4) | 1 (2.6) | 0 (0) | 150 (81.1) | 34 (18.4) | 1 (0.5) | 0.013 | |
| Fever | 37 (97.4) | 1 (2.6) | 0 (0) | 168 (90.8) | 15 (8.1) | 2 (1.1) | 0.176 | |
| Infection | 32 (84.2) | 4 (10.5) | 2 (5.3) | 180 (97.3) | 4 (2.2) | 1 (0.5) | 0.001 | |
Data are presented as No. (%). AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The prognosis
In non-operation group, the 1-year survival rate was 69.9%, the 2-year survival rate was 47.7%, and the 5-year survival rate was 31.8%. The median follow-up time was 23.5 months [95% confidence interval (CI): 8.0 to 39.0]. At the same time, in operation group, the 1-year survival rate was 94.0%, the 2-year survival rate was 79.3%, the 5-year survival rate was 65.0%, and the median follow-up time was 112.9 months. The difference in survival curves between the two groups was statistically significant (Figure 2). During the follow-up period, there were 15 and 65 patients with disease progression in non-operation group and operation group, respectively, and 6 and 16 patients were lost to follow-up (Table 5). There were 25 deaths in non-operation group (65.8%) and 75 deaths in operation group (40.5%). The chi-square test showed that the difference in mortality between the two groups was statistically significant (P=0.004). Primary tumor was the main cause of death in both groups (Table 5).
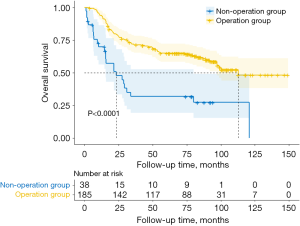
Table 5
| Follow-up results | Non-operation group (n=38) | Operation group (n=185) | P value |
|---|---|---|---|
| Disease progression | 0.612 | ||
| Locoregional progression | 5 (33.3) | 17 (26.2) | |
| Distant progression | 7 (46.7) | 40 (61.5) | |
| Overall progression | 3 (20.0) | 8 (12.3) | |
| Lost to follow-up | 6 | 16 | 0.296 |
| Alive | 7 | 94 | <0.001 |
| Dead | 0.004 | ||
| Esophagus cancer death | 18 (72.0) | 56 (74.7) | |
| Other cancer-related death | 3 (12.0) | 18 (24.0) | |
| Perioperative death | 4 (16.0) | 1 (1.3) |
Data are presented as No. (%).
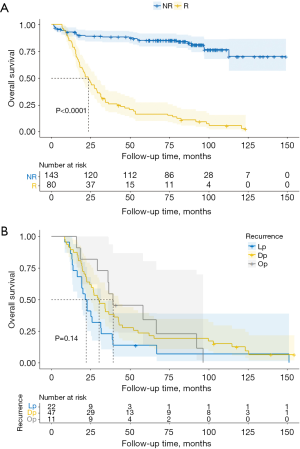
The results of multivariate analysis showed that the treatment method and recurrence had an impact on the survival of patients (Table 6), and the HR was 1.749 (P=0.018, 95% CI: 1.100 to 2.779) and 8.914 (P<0.001, 95% CI: 5.580 to 14.240), respectively. The OS of patients with recurrence was significantly lower than that of those without recurrence (Figure 3).
Table 6
| Parameter | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.020 (0.990–1.050) | 0.197 | |||
| Gender | |||||
| M | 1 | ||||
| F | 0.544 (0.290–1.019) | 0.057 | |||
| Tumor location | |||||
| Pt | 1 | ||||
| Mt | 0.868 (0.478–1.573) | 0.640 | |||
| Dt | 0.962 (0.479–1.935) | 0.914 | |||
| cT | |||||
| T1-2 | 1 | ||||
| T3 | 0.937 (0.526–1.670) | 0.827 | |||
| T4 | 1.543 (0.842–2.829) | 0.161 | |||
| cN | |||||
| N0 | 1 | ||||
| N1 | 0.820 (0.486–1.384) | 0.458 | |||
| Recurrence | |||||
| N | 1 | 1 | |||
| Y | 9.761 (6.157–15.476) | <0.001 | 8.914 (5.580–14.240) | <0.001 | |
| Treatment | |||||
| Neo-CRT + S | 1 | 1 | |||
| Neo-CRT | 2.993 (1.896–4.727) | <0.001 | 1.749 (1.100–2.779) | 0.018 | |
M, male; F female; Pt, proximal third; Mt, middle third; Dt, distal third; cT, clinical T stage; cN, clinical N stage; N, no; Y, yes; Neo-CRT + S, neoadjuvant chemoradiotherapy + surgery; Neo-CRT, neoadjuvant chemoradiotherapy; OS, overall survival; CI, confidence interval; HR, hazard ratio.
Discussion
In this retrospective analysis, the OS of patients without surgery was significantly shorter than that of patients after Neo-CRT, and the OS of patients with recurrence was shorter than that of patients without recurrence. Our results suggest that combination therapy is beneficial for patients with locally advanced ESCC. Patients with Neo-CRT alone and without surgery have poor prognosis.
Adverse events are adverse medical events that occur after patients or subjects in clinical trials receive a drug, but they do not necessarily have a causal relationship with treatment. Over the years, platinum-based therapies have been widely used in Neo-CRT for patients with ESCC and have achieved satisfactory results (4,6-9). However, the adverse reactions caused by chemotherapy have also attracted much attention. In this retrospective analysis, the most common adverse reaction after Neo-CRT was leukopenia, which is consistent with previous clinical trials of Neo-CRT for ESCC (10-12). In this data, we can clearly see that the infection rate of the 2 patient groups was significantly different, the infection rate of non-operation group was significantly higher than that of operation group. Whether the infection factor is the influencing factor of patients without surgery remains to be further studied.
According to the current data, for patients with locally advanced ESCC, the OS of patients treated with Neo-CRT combined with surgery was significantly better than that of those treated with Neo-CRT alone (without surgery), which was inconsistent with the previous literature (13). Advances in surgical techniques may be part of the reasons.
There were some limitations to this paper. Due to the retrospective analysis, the data were not sufficiently detailed for our purposes. In addition, this study did not include patients with poor general condition and those over 70 years old. Further study is required to investigate the applicability of our finding to these patients. Some patients were followed up for too short a time and their ideal outcomes may have thus been overlooked. Additionally, the two groups patients are not completely comparable and need further study.
Conclusions
To sum up, no matter in terms of recurrence rate or OS rate, the prognosis of patients in operation group was better than that in non-operation group. Therefore, Neo-CRT combined with esophagectomy is recommended for locally advanced ESCC with acceptable surgical risk.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-296/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-296/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-296/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration Helsinki (as revised in 2013). This study was approved by Medical Ethics Committee of Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University (No. K20220423). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi 2019;41:19-28. [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg 2021;156:721-9. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Hayata K, Ojima T, Nakamori M, et al. Neoadjuvant Chemotherapy with Docetaxel, Cisplatin and S-1 for Resectable Advanced Esophageal Cancer. Anticancer Res 2018;38:5267-73. [Crossref] [PubMed]
- Sasaki K, Uchikado Y, Omoto I, et al. Neoadjuvant chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-RT) for locally advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol 2019;83:581-7. [Crossref] [PubMed]
- von Döbeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus 2019; [Crossref] [PubMed]
- Wong IYH, Lam KO, Zhang RQ, et al. Neoadjuvant Chemoradiotherapy Using Cisplatin and 5-Fluorouracil (PF) Versus Carboplatin and Paclitaxel (CROSS Regimen) for Esophageal Squamous Cell Carcinoma (ESCC): A Propensity Score-matched Study. Ann Surg 2020;272:779-85. [Crossref] [PubMed]
- Hashimoto M, Shirakawa Y, Maeda N, et al. Induction chemoradiotherapy including docetaxel, cisplatin, and 5-fluorouracil for locally advanced esophageal cancer. Esophagus 2020;17:127-34. [Crossref] [PubMed]
- Boers J, Joldersma A, van Dalsen AD, et al. Intensified Neoadjuvant Chemoradiotherapy for Patients with Potentially Resectable Esophageal Cancer: A Retrospective Cohort Study. Ann Surg Oncol 2020;27:1520-8. [Crossref] [PubMed]
- Goense L, van der Sluis PC, van Rossum PSN, et al. Perioperative chemotherapy versus neoadjuvant chemoradiotherapy for esophageal or GEJ adenocarcinoma: A propensity score-matched analysis comparing toxicity, pathologic outcome, and survival. J Surg Oncol 2017;115:812-20. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
(English Language Editor: J. Jones)



