
Study on safety and efficacy of regorafenib combined with transcatheter arterial chemoembolization in the treatment of advanced hepatocellular carcinoma after first-line targeted therapy
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver and the fourth leading cause of cancer-related death worldwide (1,2). The global 5-year overall survival rate of HCC is only 20%, while the 5-year overall survival rate for advanced HCC is even lower (3). Because HCC is difficult to diagnose and highly aggressive in the early stage, most patients with HCC are already in the advanced stage at the time of diagnosis. Therefore, how to effectively treat patients with advanced HCC and prolong overall survival and progression-free survival (PFS) are the main concerns in the treatment of HCC worldwide.
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, the standard treatment for advanced HCC is systemic therapy, including transcatheter arterial chemoembolization (TACE) and chemotherapy (4). When used as a first-line therapy for the treatment of advanced HCC, TACE causes avascular necrosis of tumor cells by embolizing the tumor blood supply artery, while also increasing the concentration of local chemotherapeutic drugs. This prolongs the action time of the drug and reduces systemic side effects (5). However, TACE treatment causes tumor cells to undergo ischemic or hypoxic mutations, and the resulting expression of vascular endothelial growth factor (VEGF) and upregulation of angiogenic responses can lead to the formation of new blood vessels and ultimately cause tumor recurrence. A study has shown that the combination of TACE and anti-angiogenic drugs can reduce the expression of VEGF, reduce the formation of new blood vessels, and improve the efficacy of TACE in the treatment of HCC (6). Sorafenib, a multikinase inhibitor that blocks tumor angiogenesis and growth, was the first targeted drug used to treat advanced HCC, but its response rate and survival advantage remain unsatisfactory. Therefore, it is necessary to find new targeted drugs to improve the treatment of advanced HCC (7,8).
Regorafenib, a new oral multikinase inhibitor, was approved by the United States Food and Drug Administration (FDA) in 2017 for the second-line treatment of patients with advanced HCC after failed treatment with sorafenib. Regorafenib could inhibit VEGFR, tyrosine kinase with immunoglobulin and epidermal growth factor homology domains-2 (TIE-2), platelet-derived growth factor receptor-β (PDGFR-β), and fibroblast growth factor receptor 1 (FGFR-1) (9,10). Several clinical studies have shown that TACE combined with targeted drugs has higher efficacy in patients with advanced HCC (7,8). However, there is no research on regorafenib combined with TACE as a second-line treatment for patients with advanced HCC. The aim of this study was to investigate the efficacy and safety of regorafenib combined with TACE in the treatment of advanced HCC after the failure of first-line targeted therapy. We present the following article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-395/rc).
Methods
Patient population
This is a retrospective study and designed by our department. A total of 59 patients with advanced HCC who received second-line treatment between October 2019 and September 2021 were enrolled in the study. All patients were diagnosed with HCC by pathologists with more than 5 years of experience. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Bioethics Committee of Beijing Friendship Hospital Affiliated to Capital Medical University (No. 2021-P2-219-01). During the operation, all patients participating in this study signed informed consent. During data collection, patient records are anonymized. The inclusion criteria were: (I) age ≥18 years; (II) patients with advanced HCC received second-line regorafenib treatment; (III) patients with BCLC B/C Stage; (IV) Child-Pugh class A/B; (V) Eastern Cooperative Oncology Group (ECOG) score 0–1; (VI) At least one measurable lesion was evaluated. The exclusion criteria were: (I) diagnosis of patients with malignant tumors of other organ systems; (II) Child-Pugh class C.
We had two doctors in charge of data collation. Both doctors received more than 10 years of medical training and had rich experience. The data collected by the two doctors would be checked against each other. All patients included in the analysis obtained the following information: medical history, physical examination results, serum laboratory test results [alpha fetoprotein (AFP) level and hepatitis B virus surface antigen (HBsAg)] and radiological examination results [computed tomography (CT), magnetic resonance imaging (MRI) and/or Doppler ultrasound]. The inclusion criteria were as follows: (I) patients aged ≥18 years; (II) patients with advanced HCC who had disease progression after receiving at least 1 targeted therapy of sorafenib, lenvatinib, or apatinib with or without immunotherapy; (III) patients with BCLC stage B/C HCC; (IV) patients with Child-Pugh class A/B liver function; (V) patients with an Eastern Cooperative Oncology Group (ECOG) score of 0–1; (VI) at least 1 measurable lesion was evaluated. The exclusion criteria were as follows: (I) patients with malignant tumors of other organ systems; (II) HCC patients with Child-Pugh class C; (III) any contraindication for therapy with TACE or regorafenib.
TACE procedure
For transarterial chemoembolization, we used the Seldinger technique and introduced a 5 French RH catheter into the tumor feeding artery. Then, we carefully identified the number, location, size, and branches of the tumor. A mixture of lipiodol, epirubicin, and gelatin sponge was injected into the arterial branches. TACE was administered 1–6 times depending on the patient’s liver function and tumor shrinkage and repeated every 1 month.
Regorafenib treatment
In combination therapy, regorafenib treatment may occur during and after TACE treatment. In this study, regorafenib (40 mg/pill; Bayer HealthCare AG, Leverkusen, Germany) was administered orally 1 week after the TACE operation at a dosage of 80–160 mg once a day for the first 3 weeks of each 4-week cycle, followed by 1 week off treatment. If a grade 3 or 4 adverse event (AE) occurred, the dose of regorafenib was reduced to 40–80 mg per day and the next cycle was delayed to 28 days. If the toxicity dropped to the baseline level, the dose was increased to 80–160 mg at the discretion of the investigator. If the patient needed to reduce the dose more than 2 times or the delay between 2 cycles exceeded 28 days, the treatment of regorafenib was stopped. If the AEs had not disappeared or decreased after 1 week of adjusting the dose, the patient was advised to stop regorafenib until the symptoms were alleviated or disappeared.
Tumor response and toxicity assessment
The tumor response was evaluated by radiological examination according to the modified Response Evaluation Criteria in Solid Tumors (m-RECIST) guidelines. Complete response (CR) was defined as the disappearance of any arterial enhancement in the target tumor, partial response (PR) was defined as a >30% decrease in the sum of the diameters of viable lesions, progressive disease (PD) was defined as a >20% increase in the sum of the diameters of viable lesions, and stable disease (SD) was defined as any case that was non-PR or non-PD. The ORR was defined as the percentage of patients who achieved either CR or PR, and the disease control rate (DCR) was defined as the percentage of patients who achieved CR, PR, or SD.
Statistical analysis
Statistical analyses were performed using SPSS version 25.0 (IBM Corporation, Armonk, NY, USA). Qualitative indicators were expressed in frequency and percentage. Quantitative indicators included the number of cases, mean, standard deviation, median, minimum, and maximum. The results were expressed as a statistical table, and statistical graphs were used to provide statistical results when necessary. A t-test was used to detect measurement data, a chi-square test was used to test the comparison of count data between groups, and Fisher’s exact probability method was used when necessary. The continuous variables in our data conform to the normal distribution. A result of P<0.05 was considered statistically significant and was two-sided, and the confidence interval was 95%. Most of the analyses in our study were descriptive.
Results
Baseline characteristics
A total of 59 patients were included in the treatment population. The clinical characteristics of the patients are summarized in Table 1. The median age was 56.8 years, and 47 patients (79.7%) were male. A total of 39 patients (66.1%) were infected with the hepatitis B virus. In terms of Child-Pugh score, 38 patients (64.4%) were class A, and the remaining 21 were class B. In terms of BCLC score, 40 (67.8%) patients were stage C, and the remaining 19 were stage B.
Table 1
| Parameter | Regorafenib + TACE |
|---|---|
| Median age | 56.8 |
| Sex | |
| Male | 47 (79.7%) |
| Female | 12 (20.3%) |
| BCLC | |
| B | 19 (32.2%) |
| C | 40 (67.8%) |
| Child-Pugh | |
| A | 38 (64.4%) |
| B | 21 (35.6%) |
| AFP | |
| <400 ng/mL | 35 (59.3%) |
| ≥400 ng/mL | 24 (40.7%) |
| HBV | |
| Y | 39 (66.1%) |
| N | 20 (33.9%) |
TACE, transcatheter arterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; HBV, hepatitis B.
Efficacy
We evaluated the tumor response of TACE combined with regorafenib according to the m-RESIST criteria. The tumor responses of the patients are shown in Table 2. Among the 59 patients, 1 patient achieved CR, and 24 patients achieved PR. Fourteen patients were assessed as SD, and progressive disease was observed in 20 patients. The ORR was 42.3% (25/59), and the DCR was 66.1% (39/59). The longest follow-up was 23 months, and the shortest was 1 month. Disease progression was found in 45 patients during follow-up. Among these patients, the longest interval before the detection of disease progression was 16 months, and the shortest was 1 month. Among patients who had disease progression, the median PFS was 8 months (Figure 1). At the time of writing, there were still 14 patients didn’t find disease progression. Among these patients, the longest follow-up was 21 months.
Table 2
| Parameter | Regorafenib + TACE |
|---|---|
| CR | 1 |
| PR | 24 |
| SD | 14 |
| PD | 20 |
| ORR | 42.3% |
| DCR | 66.1% |
TACE, transcatheter arterial chemoembolization; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Safety
AEs were observed in 59 patients. These AEs included hand-foot reaction (n=50, 84.7%), weight decrease (n=18, 30.5%), hypertension (n=8, 13.6%), proteinuria (n=1, 1.7%), weakness (n=12, 20.3%), diarrhea (n=1, 1.7%), and hoarseness (n=9, 15.3%). The most common serious AE (sAE) above grade 3 according to the Common Terminology Criteria for Adverse Events (CTCAE) was hypertension. Grade 3 sAE was observed in 8 patients, and grade 4 sAE was observed in 1 patient (7%). No treatment-related deaths were observed. The cause of death for all patients who died during follow-up was the primary disease.
Discussion
The present study was the first to investigate the efficacy and safety of regorafenib combined with TACE in the treatment of patients with advanced HCC after the failure of first-line targeted treatment. Our results showed that for patients with advanced HCC, the combination of TACE and regorafenib as a second-line therapy had better efficacy than monotherapy and did not show more serious toxicity than regorafenib alone. In our study, 62.5% of patients with advanced HCC received an objective response through regorafenib combined with TACE treatment, and 81.3% of patients achieved disease control. The 3- and 6-month PFS times were 92.7% and 81.3%, respectively. During combined treatment, the most common sAE in patients above CTCAE grade 3 was hypertension, with an incidence rate of 18% (n=3, including grade 3 sAE in 2 cases and grade 4 sAE in 1 case). No treatment-related death occurred.
Regorafenib is an oral tyrosine kinase inhibitor (TKI) that inhibits a variety of enzymes such as tyrosine kinases; vascular kinases (VEGFR, PDGFR-β, and FGFR-1). Since 2017, regorafenib has been approved for clinical treatment as a second-line treatment for patients with advanced HCC, and it is advancing as a first-line targeted treatment (11). In a Phase III RESORCE trial of patients with advanced HCC, the regorafenib group showed a significant advantage in overall survival over the placebo group (10.6 vs. 7.8 months). The ORR, DCR, and PFS of patients were 11%, 65%, and 3.1 months, respectively, for patients in the regorafenib group. The most common clinically related AE of grade 3 or above was hypertension (15%, n=57) (12).
According to the Barcelona staging system, TACE is the standard treatment for intermediate HCC. TACE has proven to be an effective method for treating patients with advanced and unresolved HCC who cannot be treated with ablation or transplantation. The results of treatment assessed by the standards of the World Health Organization (WHO), the average ORR of TACE with advanced HCC was 35% (range, 16–61%) (13). A randomized controlled trial to assess the efficacy of transatlantic lipid alcohol chemotherapy in patients with volatile HCC showed that the 72% ORR in the chemotherapy group was significantly higher than the 10% ORR in the control group. However, the evaluation of the tumor response in this study was based on imaging measurements of tumor size rather than m-RESIST (14).
In HCC, normal liver tissue is mainly supplied by the inlet vein system, while tumor tissue is mainly supplied by the hepatic artery. The more advanced the tumor stage, the higher the proportion of blood supplied by the hepatic artery. TACE causes local necrosis of the tumor and protects normal liver tissue by selectively injecting chemoembolization agents into the hepatic artery. Therefore, TACE is often effective for advanced HCC (15). However, TACE causes local hypoxia of the tumor, resulting in the secretion of more VEGFs, which can eventually lead to disease progression. As a multitarget TKI, regorafenib can reduce the expression of VEGFRs after TACE, thereby improving the tumor response and reducing the recurrence rate (16). A study by Choi et al. revealed that combining another TKI (sorafenib) with TACE had a significant effect on delaying tumor progression in patients with advanced HCC, although there was no clear evidence of a survival benefit for patients (8). The study found that TACE can enhance the cytotoxic effect of TKIs on local tumor cells, thus prolonging the disease control time. In addition, low liver function is an independent risk factor for poor TACE prognosis, and TKIs combined with TACE could protect liver function by reducing the frequency of TACE. This may be why regorafenib combined with TACE is effective in patients with advanced HCC after the failure of first-line targeted therapy.
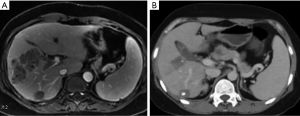
Our research suggested that patients with refractory disease after first-line targeted therapy could be expected to benefit from regorafenib combined with TACE, although further research is required to validate our trial results. In our study, there was 1 case of CR in all patients. This patient changed to second-line therapy with regorafenib and a programmed cell death 1 (PD-1) antibody rapidly after the failure of first-line targeted therapy (Figure 2). We also found that TACE combined with regofinib will prolong PFS as long as it occurs (Figure 1). Nonetheless, we recognize that this study had certain limitations. First, because our study was a retrospective study and lacked a control group or a randomized design, selection and reporting bias could not be ruled out. It should be noted that TACE is an invasive procedure and is more likely to be recommended to patients with better liver function. In this study, 64.4% of the patients had Child-Pugh class A liver function. Therefore, the effectiveness of regorafenib combined with TACE in patients with Child-Pugh class B liver function requires further verification. Second, this study was a small, single-center sample study, which decreases its statistical credibility. Therefore, the results may not be extended to other countries. Finally, the dosage of embolic and chemotherapeutic agents in TACE was determined by the attending physician, and there are no standards or subjective criteria.
Conclusions
This study retrospectively analyzed the efficacy and safety of regorafenib combined with TACE in the treatment of patients with advanced HCC after the failure of first line targeted therapy. The results showed that combination therapy had better therapeutic effect and safety than regorafenib monotherapy. Therefore, patients with refractory advanced HCC could benefit from the treatment of regorafenib combined with TACE.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-395/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-395/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-395/coif). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020;31:334-51. [Crossref] [PubMed]
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021;73:4-13. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127:S179-88. [Crossref] [PubMed]
- Ranieri G, Ammendola M, Marech I, et al. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J Gastroenterol 2015;21:6018-25. [Crossref] [PubMed]
- Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. [Crossref] [PubMed]
- Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 2013;269:603-11. [Crossref] [PubMed]
- Rimassa L, Pressiani T, Personeni N, et al. Regorafenib for the treatment of unresectable hepatocellular carcinoma. Expert Rev Anticancer Ther 2017;17:567-76. [Crossref] [PubMed]
- Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012;18:2658-67. [Crossref] [PubMed]
- Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 2013;12:1322-31. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Lee HS, Kim JS, Choi IJ, et al. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer 1997;79:2087-94. [Crossref] [PubMed]
- Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol 2013;24:2565-70. [Crossref] [PubMed]
- Asayama Y, Yoshimitsu K, Nishihara Y, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol 2008;190:W28-34. [Crossref] [PubMed]
- Bao Y, Feng WM, Tang CW, et al. Endostatin inhibits angiogenesis in hepatocellular carcinoma after transarterial chemoembolization. Hepatogastroenterology 2012;59:1566-8. [Crossref] [PubMed]



