Correlation analysis of intestinal flora and immune function in patients with primary hepatocellular carcinoma
Introduction
Primary liver cancer is the fourth most common malignant tumor and the second most lethal cause of cancer in China, which seriously threatens the life and health of Chinese people (1). It mainly includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and HCC-ICC mixed pathologic types. HCC accounts for 85–90% of cases, so the term “liver cancer” in this study refers to HCC (2). Since the early clinical symptoms of HCC patients are not obvious, most patients are diagnosed with advanced HCC (3). Therefore, there is a pressing need to identify specific markers for the diagnosis of HCC (4). The intestinal tract and liver are both crucial for nutritional absorption. Intestinal flora can interact with the liver through the “enteric-liver axis” (5). The imbalance of intestinal flora and the increase of intestinal mucosal permeability will lead to the bacteria and bacterial components, such as lipopolysaccharide (LPS) and deoxyribonucleic acid (DNA), which are collectively referred to as the pathogen-associated molecular pattern (PAMP) (6-8). Pathogen-associated molecular pattern (PAMPAs) are recognized by immune receptors on liver Kupffer cells and hepatic stellate cells to initiate and maintain inflammation, and this cascade leads to fibrosis and damage to the liver, which continues to develop into liver cancer (9).
Hepatoenteric circulation and a normal intestinal mucosal barrier are necessary conditions to maintain stability in the body (10). Intestinal flora imbalance and leaky gut are two of the most important findings in liver cancer patients, reflecting the possible role of intestinal flora in the development of the disease (11). In this study, fecal flora analysis and metagenomic correlation analysis were performed on patients with liver cirrhosis and healthy people, and the characteristic intestinal flora and related gene profiles were obtained. It is believed that the significant change in intestinal flora composition in patients with liver cirrhosis has a certain clinical diagnostic value (12). Bacterial imbalance and intestinal leakage caused by various chronic pathogenic factors such as viral infection, alcohol, and metabolic abnormalities lead to direct contact between the liver and intestinal flora and their metabolites (13). It is believed that immunity and metabolism are the main mechanisms of interaction between the liver and microflora. PAMPs are the theoretical basis for the influence of intestinal flora on the development of HCC through mediated immune pathways. Intestinal flora and its related products can be recognized by the immune system and induce a series of immune responses (14).
Liver disease is often associated with increased intestinal permeability. Disruption of the intestinal barrier allows the transfer of microbial products and live bacteria from the intestinal lumen to the extra-intestinal organs. Most of the venous blood from the intestine flows into the portal vein circulation, which is part of the blood supply to the double liver. As a result, the liver is the first organ in the body to encounter not only absorbed nutrients, but also enteric-borne bacterial and pathogen-associated molecular patterns. Long-term exposure to high levels of pathogen-associated molecular patterns is associated with early disease progression and infectious complications of advanced liver disease (cirrhosis). The anatomy of the liver and gastrointestinal tract is closely related and is known as the enteric-liver axis. The liver receives nearly 70 percent of the intestinal blood through the portal vein, and most metabolites from the intestine pass through the liver. These metabolites include nutrients, gut bacteria and bacterial metabolites such as toxins, DNA and cell membrane fragments produced by bacterial death. So the liver is both the first barrier to gut derived antigens and the organ most exposed to enterotoxins. Intestinal endotoxemia (IETM) caused by bacterial overgrowth, bacterial translocation and intestinal mucosal permeability changes plays a key role in the occurrence and development of NASH. This is because the liver is also an immune organ, and although the imbalance of the flora is not an infection, the immune system will also be activated and initiate an immune inflammatory response, thus causing damage to its own tissues. NASH can progress to fibrosis, cirrhosis, and even liver cancer. It is worth noting that intestinal microflora disorder is an interactive process with the occurrence and development of liver cancer, and the progression of liver cancer may further aggravate intestinal microflora disorder. Abnormal liver function and portal hypertension are common complications in the progression of liver cancer. When the liver function declines, on the one hand, the synthesis of bile acid is reduced, resulting in the weakening of the inhibitory effect of bile acid on intestinal harmful bacteria; on the other hand, the function of KCs in the liver to clear bacteria entering the portal vein system and its metabolic product LPS is decreased, and endotoxemia is easy to occur. At the same time, portal hypertension can cause intestinal mucosal congestion edema, increased permeability, resulting in intestinal mucosal barrier damage. 16SrDNA amplicon sequencing detected that the intestinal flora of liver cancer patients in this experiment was changed compared with that of healthy volunteers. Bioinformatics methods were used to predict the metabolic functions of the community samples. The abundance of genes encoding some metabolic functions (nucleotide metabolism, amino acid metabolism, lipid metabolism, energy metabolism and carbohydrate metabolism, etc.) as well as transmembrane transport, replication and translation was significantly higher in the control group than in the experimental group. The expression of IL-6 and IL-10 in serum of the experimental group was higher than that of the control group, and the difference was statistically significant, while il-12 was the opposite.
In this study, patients with primary liver cancer were selected as the study subjects, and healthy individuals were included as controls. Metagenomic sequencing technology was used to explore the intestinal microflora distribution and immune function characteristics of patients with primary liver cancer. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-427/rc).
Methods
Study outline
A total of 10 patients with primary liver cancer (primary liver cancer group) and 10 healthy subjects (healthy control group) who were admitted to The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University from March to June 2021 were retrospectively selected as the research objects. Primary liver cancer diagnosis criteria refer to the primary liver cancer diagnosis and treatment guidelines updated in 2021. The healthy subjects were in good physical condition, had no drinking habits, and their genders and ages were matched with the primary liver cancer patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University (No. 20210012). Individual consent for this retrospective analysis was waived.
Inclusion criteria
The inclusion criteria were as follows: (I) body mass index (BMI) of 20–30 for males and 19–34 for females; (II) complete liver function, biochemistry, tumor serological indexes, and imaging examination data; (III) patients with no history of autoimmune diseases or diabetes; (IV) those with no history of using antibiotics or therapeutic probiotics in the past 8 weeks; (V) patients who denied a history of other infectious diseases; (VI) those with no intestinal diseases or other systemic diseases; (VII) patients without any special eating habits (such as vegetarian diet, etc.); and (VIII) non-pregnant or breastfeeding women.
Research method
Sample collection and fecal DNA extraction
Stool specimens from healthy controls and primary liver cancer patients were confirmed in the hospital for the first time. the specimens to return to choose huada stool specimens collection of supports, suits, samples sent to Beijing genomics co., Ltd. for DNA extraction: specimens after centrifugal grinding, cracking, incubation of precipitation. Following high-speed centrifugation, total DNA was obtained by washing, incubation, and elution.
Metagenomic DNA library construction and sequencing process
(I) DNA fragments were purified by breaking and repairing the qualified DNA samples; (II) the adaptors were connected at both ends of the DNA fragments, and polymerase chain reaction (PCR) amplification was performed to recover the target fragments; (III) the sample library was controlled and quantified; and (IV) eligible libraries were sequenced using the Illumina platform (American).
Similarity analysis
Principal components analysis (PCA) uses linear transformation to convert the data into a new coordinate system, and then applies the idea of dimension reduction to place the largest variance of any data projection on the first coordinate and the second-largest variance on the second coordinate (the second principal component). PCA can reduce the dimensionality of the dataset, while also maintaining the largest contribution of the variance of the dataset, and finally visually present the data in the two-dimensional coordinate system.
Diversity analysis
Beta diversity uses the evolutionary relationship and abundance information between each sample sequence to calculate the inter-sample distance, reflecting whether there are significant microbial community differences between the sample groups. Metagenomic projects typically apply the Bray-Curtis and Jaccard distances to measure the differences between groups.
Species abundance analysis
The alignment results were processed using MEGAN (Version 4.6). MEGAN recombines the basic local alignment search tool (BLAST) alignment results from an algorithm called the least common ancestor (LCA). Thus, the species annotation information for each sequence. Gene abundances annotated by the same species were summarized to obtain in the sample species abundance.
Clinical indicators
BMI and ALT were measured in the healthy controls and primary liver cancer patients. We also analyzed the patients’ ALT, Aspartate aminotransferase (AST), γ-glutamyl transferase (GLUTamyl transferase, GGT), total protein, total bilirubin, and alpha-fetoprotein (AFP) levels, and performed abdominal ultrasounds. The dietary habits and medication history of the patients were recorded during the first 8 weeks of enrollment. A balanced diet was considered to include vegetarian and meat.
Statistical analysis
Data analysis was performed with SPSS 22.0 and GraphPad Prism 7.0 software, and mapping was completed using R 3.6.0 software (American). Normally-distributed age, BMI, ALT, AST, total protein, and total bilirubin level measurement data were presented as , and the independent sample t-test was used for comparisons between the two groups. GGT, AFP, and tumor size were presented as median (quartile). Gender distribution was presented as count data, and comparisons between the two groups were performed using the Fisher’s exact probability method test. Streptococcus mutans, Streptococcus thermophilus, H. parainfluenzae, VerRong, Ackeria, Prevotella, and other bacteria, as well as serum levels of ALT, AST, GGT, total bilirubin, total protein, and AFP, were respectively examined by Spearman analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Baseline data of the included study subjects
A total of 20 samples were collected, including 10 from healthy controls and 10 from primary liver cancer patients. In the primary liver cancer group, there were nine males and one female, with an average age of 53.9±6.9 years. In the healthy control group, there were nine males and one female, with an average age of 56.4±13.2 years. These differences were not statistically significant (P>0.05), indicating comparability between the groups. Compared with the healthy control group, GGT and AFP levels in the HCC group were significantly increased (Z=2.937, P=0.002, Z=2.531, P=0.010).
Correlation analysis of intestinal microflora
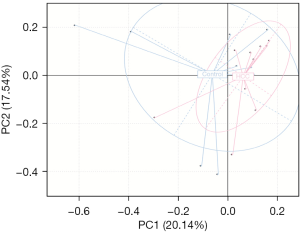
PCA uses the idea of dimensionality reduction to extract the most important elements and structures from multi-dimensional data. The closer the distance between two groups of samples in the figure is, the more similar the composition and structure of the samples are. In the PCA of this study, each point represented a specimen, and it was seen that the two groups exhibited different bacterial community structures. The healthy control group was more dispersed, while the primary liver cancer group was more concentrated, but the two groups were not completely separated in the direction of PC1 and PC2, suggesting that the composition and structure of the two groups were similar to a certain extent (Figure 1).
Analysis of gut microbiota diversity among the study subjects
Beta diversity analysis was used to compare differences in species diversity between sample groups and was measured by calculating the Bray and JSD distances of samples according to the abundance matrix. The difference between the two groups was statistically significant (P<0.001). Furthermore, there was also a statistically significant difference in the species level Beta diversity analysis between the healthy control and primary liver cancer groups, and fecal microbial diversity in the primary liver cancer group was lower than that in the healthy control group (Figure 2).
Correlation analysis between intestinal flora and immune score
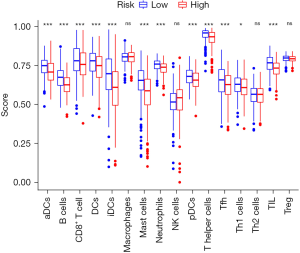
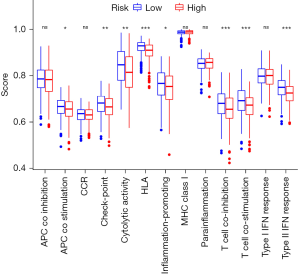
Both the innate and adaptive immune responses may promote or inhibit HCC development, partly because HCC often occurs in the context of chronic inflammation. Although immune cells are key factors in immune surveillance, they may also induce HCC in Chronic Liver Disease (CLD) by promoting inflammation (Figure 3). In addition, the tumor inhibition of B and T cells via injection of diethylnitrosamine (DEN). In contrast, CD8+ T cells have been shown to promote liver inflammation, leading to nonalcoholic steatohepatitis (NASH). Liver immunosurveillance involves different cell populations that are associated in specific ways. In the context of NASH, CD4+ T cells have also been shown to play an important role in HCC monitoring (Figure 4).
Analysis of intestinal microflora abundance
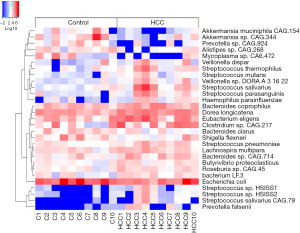
A total of 956 genera and 3,436 species were identified from the 20 specimens. At the genus level, the abundance of Bacteroides was the highest with a relative abundance of 34.94% (11.76%, 56.02%), followed by Prevotella with a relative abundance of 11.99% (1.29%, 27.82%). At the species level, the highest abundance of Bacteroides vulgatus and Faecalibacterium prausnitzii were 1.55% (0.63%, 3.90%) and 1.54% (0.53%, 2.84%), respectively. The results also showed that the distribution of 137 species in the healthy control and primary liver cancer groups were significantly different (P<0.05). The abundance of Aikman, Akkermansia muciniphila, Prevotella, and Branchella decreased significantly (Figure 5).
Discussion
The early symptoms of primary liver cancer patients are not obvious, and there is currently a lack of specific markers for early diagnosis (15). Most patients with primary liver cancer are already in the advanced stage upon diagnosis, and the prognosis is poor. The interaction between gut microbes and the liver through the “gut-liver axis” makes it possible to use gut microbes as diagnostic markers for liver cancer. The commonly used methods to study intestinal flora include 16S amplification sequencing and metagenomic sequencing (16). 16S sequencing is only aimed at the polymorphism of the variable region sequence of 16S ribosomal deoxyribonucleic acid (rDNA) for sequence differentiation and the identification of bacterial species.
Metagenomics refers to the whole-genome sequencing of all microorganisms (17). While most sequences obtained by 16S sequencing cannot be annotated at the species level, metagenomic sequencing can identify microorganisms down to the species level and even strains level (18-20). Therefore, metagenomic sequencing is highly advantageous for species identification. The application of intestinal flora in the early prevention and adjuvant clinical treatment of high-risk patients is another major direction of clinical application (21). On the one hand, starting from the molecular mechanism of intestinal flora involvement in the occurrence of liver cancer, the development of related target drugs, including toll-like receptors (TLR) antagonists, farnesoid X-activated receptor (FXR) agonists, and related bacterial metabolite inhibitors, for the early prevention of intestinal flora in high-risk groups is the main idea of clinical transformation of intestinal flora, and relevant target drugs have been clinically studied (22-24). On the other hand, probiotics and microbiota transplantation are direct methods of regulating intestinal microbiota. In a previous study based on animal models, the size and number of tumors in HCC mice treated with mixed probiotics were significantly reduced, accompanied by improvement in the intestinal microflora imbalance and immune microenvironment changes (25). This was specifically reflected in the increase of beneficial bacteria and inhibition of the increase of inflammatory metabolites. Chronic inflammation is the main factor inducing primary liver cancer. The occurrence of chronic hepatitis is related to the following stimuli and changes: for example, immune cell activation caused by viral infection; chronic liver injury releases proinflammatory factors and causes activation of innate immune cells. Exogenous toxins, fatty acid-mediated lipotoxicity, and iron over deposition lead to changes in hepatocyte types including hepatocytes and sinusoidal endothelial cells (LSECs). Hepatocyte death is a process associated with chronic liver diseases such as NASH, viral hepatitis, and cirrhosis, and includes three forms of apoptosis, necrotic apoptosis, and necrosis. The type of cell death affects the type of primary liver cancer.
NASH has been shown to induce linoleic acid accumulation, which in turn leads to reactive oxygen species-mediated CD4+ T cell death, leading to accelerated tumor growth (26). The role of B cells in the immunological surveillance of HCC has been demonstrated in both in situ analysis of the interaction between B cells and T cells in human samples as well as mouse studies, while accumulation of CD4+ regulatory T cells and myeloid suppressor cells has been shown to damage effector T cells and natural killer (NK) cells (27). In this study, metagenomic sequencing was used to explore the characteristic changes of intestinal flora in patients with primary HCC, and specific differential species were identified, indicating their potential use as markers of HCC (28-30). However, certain metabolites of intestinal flora may protect liver function and play a positive role in preventing liver cancer, such as indolepropionic acid, a bacterial breakdown product of tryptophan that inhibits NF-κB signaling, reduces levels of pro-inflammatory cytokines, and antagonizes liver inflammation and liver damage. In conclusion, intestinal flora is closely related to the occurrence and development of liver cancer.
Therefore, intestinal flora may be used as a biomarker for the early diagnosis of primary liver cancer. The application of the intestinal microbiome as a marker, combined with the current diagnostic methods for HCC, may further facilitate the early diagnosis and treatment of HCC. In addition, based on the characteristics of intestinal flora in patients with primary liver cancer, the different bacteria involved in the flora imbalance can provide new ideas for the development of probiotics in the future.
Acknowledgments
Funding: The study was funded by the project of Changzhou medical innovation team (No. CCX201807).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-427/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-427/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-427/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University (No. 20210012). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yuen VW, Wong CC. Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Invest 2020;130:5052-62. [Crossref] [PubMed]
- Heinrich S, Craig AJ, Ma L, et al. Understanding tumour cell heterogeneity and its implication for immunotherapy in liver cancer using single-cell analysis. J Hepatol 2021;74:700-15. [Crossref] [PubMed]
- Feng GS, Hanley KL, Liang Y, et al. Improving the Efficacy of Liver Cancer Immunotherapy: The Power of Combined Preclinical and Clinical Studies. Hepatology 2021;73:104-14. [Crossref] [PubMed]
- Lurje I, Hammerich L, Tacke F. Dendritic Cell and T Cell Crosstalk in Liver Fibrogenesis and Hepatocarcinogenesis: Implications for Prevention and Therapy of Liver Cancer. Int J Mol Sci 2020;21:7378. [Crossref] [PubMed]
- Wang C, Cao Y, Yang C, et al. Exploring liver cancer biology through functional genetic screens. Nat Rev Gastroenterol Hepatol 2021;18:690-704. [Crossref] [PubMed]
- Gu Y, Wang Y, He L, et al. Circular RNA circIPO11 drives self-renewal of liver cancer initiating cells via Hedgehog signaling. Mol Cancer 2021;20:132. [Crossref] [PubMed]
- Ostroumov D, Duong S, Wingerath J, et al. Transcriptome Profiling Identifies TIGIT as a Marker of T-Cell Exhaustion in Liver Cancer. Hepatology 2021;73:1399-418. [Crossref] [PubMed]
- Zhang W, Zeng B, Hu X, et al. Oncolytic Herpes Simplex Virus Type 2 Can Effectively Inhibit Colorectal Cancer Liver Metastasis by Modulating the Immune Status in the Tumor Microenvironment and Inducing Specific Antitumor Immunity. Hum Gene Ther 2021;32:203-15. [Crossref] [PubMed]
- Roy S, Banerjee P, Ekser B, et al. Targeting Lymphangiogenesis and Lymph Node Metastasis in Liver Cancer. Am J Pathol 2021;191:2052-63. [Crossref] [PubMed]
- Ge Y, Zhang Z. Effect of Tumor Red Blood Cell Immunity and Tumor Cell Cycle in Mice Bearing Solid Liver Cancer with Intelligent Cancer Zhongning Therapeutic Apparatus. J Healthc Eng 2021;2021:3329800. [Crossref] [PubMed]
- Pan J, Xu Y, Wu Q, et al. Mild Magnetic Hyperthermia-Activated Innate Immunity for Liver Cancer Therapy. J Am Chem Soc 2021;143:8116-28. [Crossref] [PubMed]
- Nault JC, Cheng AL, Sangro B, et al. Milestones in the pathogenesis and management of primary liver cancer. J Hepatol 2020;72:209-14. [Crossref] [PubMed]
- Yang L, He Y, Zhang Z, et al. Upregulation of CEP55 Predicts Dismal Prognosis in Patients with Liver Cancer. Biomed Res Int 2020;2020:4139320. [Crossref] [PubMed]
- Ohtani N, Hara E. Gut-liver axis-mediated mechanism of liver cancer: A special focus on the role of gut microbiota. Cancer Sci 2021;112:4433-43. [Crossref] [PubMed]
- Meng J, Wang L, Hou J, et al. CCL23 suppresses liver cancer progression through the CCR1/AKT/ESR1 feedback loop. Cancer Sci 2021;112:3099-110. [Crossref] [PubMed]
- Wu ZJL, Li K, Zhang K, et al. Research progress of immunotherapy alone and in combination for liver cancer. Zhonghua Gan Zang Bing Za Zhi 2020;28:471-4. [PubMed]
- Bao MH, Wong CC. Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells 2021;10:1715. [Crossref] [PubMed]
- Zhu J, Wang Y, Yang P, et al. GPC3-targeted and curcumin-loaded phospholipid microbubbles for sono-photodynamic therapy in liver cancer cells. Colloids Surf B Biointerfaces 2021;197:111358. [Crossref] [PubMed]
- Fujita M, Yamaguchi R, Hasegawa T, et al. Classification of primary liver cancer with immunosuppression mechanisms and correlation with genomic alterations. EBioMedicine 2020;53:102659. [Crossref] [PubMed]
- Wang H, Li X, Peng R, et al. Stereotactic ablative radiotherapy for colorectal cancer liver metastasis. Semin Cancer Biol 2021;71:21-32. [Crossref] [PubMed]
- Jianzhu B, Shuang L, Pengfei M, et al. Research on Early Warning Mechanism and Model of Liver Cancer Rehabilitation Based on CS-SVM. J Healthc Eng 2021;2021:6658776. [Crossref] [PubMed]
- Prizment AE, Onyeaghala GC, Church TR. Letter: synergistic role of gut flora with aspirin to prevent colorectal cancers-authors' reply. Aliment Pharmacol Ther 2020;52:1758. [PubMed]
- Konrad L, Andersen K, Kesper MS, et al. The gut flora modulates intestinal barrier integrity but not progression of chronic kidney disease in hyperoxaluria-related nephrocalcinosis. Nephrol Dial Transplant 2020;35:86-97. [PubMed]
- Liu H, Cai Z, Wang F, et al. Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora. Adv Sci (Weinh) 2021;8:e2101619. [Crossref] [PubMed]
- Xue M, Liang H, Ji X, et al. Effects of fucoidan on gut flora and tumor prevention in 1,2-dimethylhydrazine-induced colorectal carcinogenesis. J Nutr Biochem 2020;82:108396. [Crossref] [PubMed]
- Sharma P, Agrawal A. Does modern research validate the ancient wisdom of gut flora and brain connection? A literature review of gut dysbiosis in neurological and neurosurgical disorders over the last decade. Neurosurg Rev 2022;45:27-48. [Crossref] [PubMed]
- Xing JW, Chen MM, Tian XY, et al. 919 syrup inhibits ROS-mediated leptin-induced anorexia by activating PPAR and improves gut flora abnormalities. Biomed Pharmacother 2021;138:111455. [Crossref] [PubMed]
- Guo J, Tang J, Kang T, et al. Different immunization methods lead to altered gut flora and varied responses to Mycobacterium tuberculosis infection in mice. J Infect Dev Ctries 2020;14:1170-7. [Crossref] [PubMed]
- Zhou Y, Qi S, Meng X, et al. Deoxynivalenol photocatalytic detoxification products alleviate intestinal barrier damage and gut flora disorder in BLAB/c mice. Food Chem Toxicol 2021;156:112510. [Crossref] [PubMed]
- Khan H, Miao X, Liu M, et al. Behavior of last resort antibiotic resistance genes (mcr-1 and blaNDM-1) in a drinking water supply system and their possible acquisition by the mouse gut flora. Environ Pollut 2020;259:113818. [Crossref] [PubMed]
(English Language Editor: A. Kassem)