
Fourth-line rescue treatment ripretinib of advanced small intestine gastrointestinal stromal tumors who achieved partial response: a case report
Introduction
Gastrointestinal stromal tumors (GISTs) originate from the interstitial cells of Cajal (ICCs), which are the pacemakers of peristaltic movement of the muscularis propria of the gastrointestinal tract and its surrounding myenteric plexus. As the most common mesenchymal tumor of the gastrointestinal tract (1), GISTs can occur in the esophagus, stomach, small intestine, colon, rectum, mesentery, and omentum, but occur most often in the stomach (2). The pathogenesis of GISTs is related to the activation of tyrosine kinase receptors caused by mutations in the Kit/PDGFRA genes. GISTs have unique biological characteristics. They can be benign tumors or spread rapidly and cause death (3). The vast majority of patients with advanced GISTs have missed the opportunity for surgery, and thus, selecting a feasible targeted therapy is crucial. As a landmark targeted drug, imatinib has achieved satisfactory efficacy in the treatment of advanced GISTs, but its roles in the second- and third-line settings are not satisfactory. Thanks to the advances in molecular biology, ripretinib has been approved as a fourth-line therapy for advanced GISTs. Ripretinib, a broad-spectrum KIT (cell growth factor receptor Kit)/PDGFRA (platelet-derived growth factor) inhibitor, has shown high efficiency in inhibiting GIST cell lines with a range of genetic mutations in vitro study (4). In a completed Phase III randomized controlled trial of the fourth-line treatment of metastatic GIST, ripretinib treatment achieved a progression-free survival (PFS) of 6.3 months, which was significantly longer than that (1.0 months) in the placebo group (5). In this paper, the case has a good response to repatinib, and the tumor long diameter have been reduced by more than 75%. At present, there is no similar report in China. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-534/rc).
Case presentation
On February 15, 2015, a 49-year-old male patient visited a subordinate hospital complained of abdominal pain for 1 month and aggravated for 3 days. Diabetes has a history of 10 years, with insulin injection for control, good blood glucose control and normal health. Deny chronic history of hypertension, coronary heart disease, hepatitis, tuberculosis and other infectious diseases. Denied the history of surgery and major trauma. There is no history of living in the epidemic area, smoking or drinking. Ultrasonography revealed a space-occupying lesion posterior to the bladder, computed tomography (CT), showed pelvic effusion, and diagnostic abdominal puncture revealed uncoagulated blood (tumor rupture). A surgical plan was then proposed but was refused by the patient because he felt that the abdominal pain had been relieved.
On February 22, 2015, he was then admitted to another hospital and received routine physical examinations, which showed that he was conscious and alert, along with a fair general condition and intermediate nutritional status. He entered the ward without any escort and was cooperative during physical examinations. There was no yellowing of the eyes and skin. His pupils were equal, round, and reactive to light. The trachea was at the midline. His breath was stable and there was no pleural friction fremitus or voice tremor. The cardiopulmonary rhythms were regular. Physical examination of the abdomen revealed the presence of symmetrical abdominal bulges. No intestinal peristaltic waves were observed. Abdominal breathing was slightly limited. There was no tenderness or rebound tenderness. While there was no palpable liver or spleen below the ribs, an obvious mass was palpated in the lower abdomen. Palpation of the liver area was negative for tenderness, and the Murphy’s sign was negative. Physical examination of the nervous system showed no obvious abnormality.
On 3rd of March, 2015, exploratory laparotomy was then performed. Intra-abdominal haemorrhage (about 500 mL in volume) was found during intraoperative exploration. The greater omentum was covered with tumor nodules of different sizes. Several pelvic tumors were also found, with the largest being 5.0 cm in diameter. These pelvic tumors had ruptured and hemorrhaged. Since R0 resection was not possible, tumors in the small bowel and pelvic region were removed (R2).
On March 8, 2015, postoperative pathology confirmed the lesions as GISTs. The specimens included a small intestine tumor (6 cm × 5 cm × 3.5 cm, with capsule), an intra-abdominal mass (11 cm × 9 cm × 3.5 cm for an irregularly-shaped tissue; the largest of which had a 6 cm × 4 cm × 4 cm capsule), and abdominal wall nodules. The cells were spindle-shaped, with 5–10 mitotic cells/50 HPF (High power field), and the results of the immunohistochemical analysis were as follows: CD117 (+), DOG-1 (+), CD34 (+), and Ki67 about 20%. Genetic testing was not performed. Postoperatively, the patient received constant treatment with oral imatinib 400 mg/d, and the best response was stable disease (SD). He had not received any surgery or experienced major trauma. Also, he had no major medical histories, such as chronic diseases (e.g., hypertension and diabetes), familial genetic diseases, or psychological diseases.
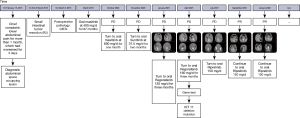
In October 2020, re-examination with CT revealed progressive disease (PD), and imatinib 600 mg was administered. In November 2020, re-examination with CT still revealed PD, and sunitinib 37.5 mg (continuously once-daily) was applied. On January 26, 2021, the patient visited our center and was diagnosed with small intestine GIST with diffuse metastases. His medical regimen was adjusted according to the CT findings (Figure 1), which included: abdominal and pelvic effusions; large space-occupying lesions in the abdominal and pelvic regions (largest cross-section: 20.4 cm × 12.5 cm; longitudinal diameter: about 28.7 cm); peritoneal thickening; multiple small nodules; and enlarged bilateral diaphragmatic lymph nodes. All these findings were suggestive of PD. The therapy was then switched to regorafenib (120 mg daily for 3 weeks followed by 1 week off schedule) (Figure 2).
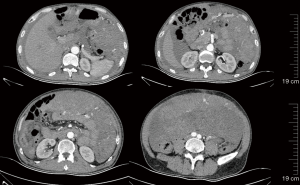
On March 13, 2021, re-examination with CT showed a large space-occupying lesion in the abdominal and pelvic region, with slightly smaller involvement than before, and the bilateral superior phrenic lymph nodes were enlarged. The clinical response was regarded as a partial response (PR). Regorafenib therapy (120 mg daily for 3 weeks followed by 1 week off schedule) continued (Figure 3).
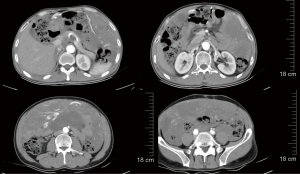
On April 27, 2021, re-examination with CT showed that the scope of the abdominal and pelvic space-occupying lesions was slightly larger than before, the volume of abdominal and pelvic effusions was slightly increased, and the number of multiple space-occupying masses in the diaphragm and cardiophrenic angle increased, indicating PD. Subsequently, the daily dose of regorafenib was increased to 160 mg (Figure 4).
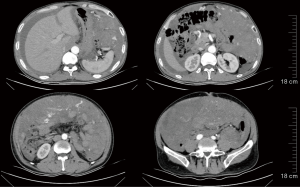
Genetic testing using the patient’s original surgical specimen detected a KIT exon 11 deletion (p.K558_I563del), although no other clinically relevant genetic mutations were detected.
On July 30, 2021, re-examination with CT showed that the scope of abdominal and pelvic space-occupying lesions was enlarged and extended to the scrotum, along with increased ascites volume, increased number and size of multiple metastatic lesions in the diaphragm muscle, cardiophrenic angle, and retroperitoneal area, indicating PD. Ripretinib 150 mg qd (quaque die) was administered (Figure 5).
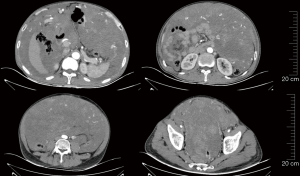
On September 27, 2021, i.e., two months after the use of ripretinib 150 mg qd, re-examination with CT showed that the abdominal and pelvic space-occupying lesions shrank, the abdominal and pelvic effusions were reduced, the widening of the right inguinal canal was alleviated, and the internal density was lower. Thus, the response to treatment was evaluated as PR. The treatment with ripretinib 150 mg qd continued (Figure 6).
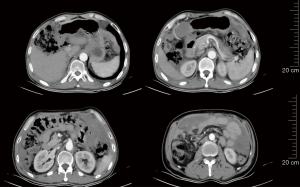
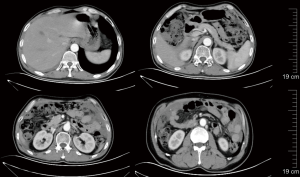
On January 18, 2022, re-examination with CT showed that the abdominal and pelvic space-occupying lesions shrank further, the abdominal and pelvic effusions were reduced, some of the multiple nodules at the cardiophrenic angle became smaller, the widening of the right inguinal canal was alleviated, and peritoneal thickening was relieved. Again, the response to treatment was evaluated as PR. The treatment with ripretinib 150 mg qd continued (Figure 7). After using Repatinib for 10 months, the patient had no other obvious side effects except hair loss.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Imatinib is the first-line treatment for metastatic GISTs, and more than 80% of GIST patients may benefit from first-line treatment with imatinib (6,7). For patients with PD after 6 months of imatinib treatment, genetic testing should be performed to identify the genotype. Secondary mutations are common and multiple in imatinib-resistant GIST patients and are the main cause of PD. Conventional TKIs (Tyrosine kinase inhibitors) are only effective for some types of mutations (8,9). Imatinib dose escalation or switching to second-line to sunitinib may be applied after the failure of standard-dose imatinib. In the real world, late-line treatment often requires dose and schedule adjustments, and drug withdrawal is common. Third-line regorafenib therapy can be selected in patients with GISTs who have failed treatment with both imatinib and sunitinib. Research has shown that the efficacy and safety of second- and third-line drugs targeting primary KIT exon 11 mutations are unsatisfactory (10). However, for patients with GISTs who have failed imatinib, sunitinib, and regorafenib, fourth-line ripretinib therapy can be selected. Secondary mutations mainly lead to structural changes in the adenosine triphosphate (ATP)-binding region or the activation loop. Ripretinib is a novel TKI inhibitor specially developed to overcome drug resistance. It can act on both the activation loop and the switch pocket, thus more broadly inhibiting the activation of signaling pathways (4).
In our current case, the patient was initially treated with oral imatinib 400 mg/d after tumor resection (R2), and SD was achieved. 67 months later, CT suggested PD, and the patient was switched to oral imatinib 600 mg/d. One month later, re-examination with CT still revealed PD, and second-line sunitinib 37.5 mg (continuously once-daily) was applied. One month after this, re-examination with CT still revealed PD, and the therapy was switched to regorafenib (120 mg daily for 3 weeks followed by 1 week off schedule). Two months later, re-examination with CT showed PR, and regorafenib therapy (120 mg daily for 3 weeks followed by 1 week off schedule) continued. One month after this, re-examination with CT showed PD, and the dose of regorafenib was increased (160 mg daily for 3 weeks followed by 1 week off schedule). Genetic testing was performed on the specimen collected during the first surgery, and no other clinically relevant mutation was detected except for a KIT exon 11 deletion (p.K558_I563del). Three months later, re-examination with CT revealed PD, and fourth-line ripretinib 150 mg/d was applied. Two months later, re-examination with CT showed that the abdominal and pelvic space-occupying lesions had shrunk, and the response to treatment was evaluated as PR. Thus, the treatment with oral ripretinib 150 mg qd continued.
In our current case, PD occurred during imatinib treatment, and the disease progressed rapidly during the second- and third-line treatments. Fortunately, PR was achieved during the fourth-line treatment, demonstrating the good efficacy of ripretinib in a fourth-line setting.
The type of secondary gene mutation in this patient was unclear, although the primary mutation was a KIT exon 11 deletion. Since the patient refused to undergo a needle biopsy and the accuracy of liquid biopsy was unsatisfactory, genetic testing was performed on the specimen collected during the first surgery. In fact, the real-world detection rate of secondary mutations remains low in China. Therefore, choosing the appropriate drug according to the type of primary mutation is particularly important. Research has shown that the efficacy of second- and third-line drugs targeting primary KIT exon 11 mutations is unsatisfactory (10). After imatinib resistance, dose and schedule adjustments are often required during second- and third-line treatments (11,12).
Imatinib, sunitinib, and regorafenib are all designed to compete at the ATP-binding site. When secondary mutations occur, whether TKI can effectively bind to the ATP-binding site depends on the difference in drug structure and binding mode. Acting on the final step of kinase activation, ripretinib binds to both the switch pocket and activation loop, thus stabilizing the kinase in its inactive conformation. Unlike other TKI1s that act on the ATP-binding site, ripretinib acts uniquely in the switch pocket, which enables it to broadly inhibit KIT and PDGFRA mutations (13). In fact, ripretinib can inhibit a broad spectrum of primary and secondary drug-resistant KIT and PDGFRA variants (4).
Based on the phase 3 INVICTUS study, regorafenib has been approved as a fourth-line treatment for advanced GISTs, and ripretinib has shown benefits within all mutation subgroups examined across both primary and secondary KIT mutations (5). Comparisons between a bridging study in China on ripretinib versus the INVICTUS study showed interesting results. All of the enrolled patients had received late-line therapies and had poor prognoses. The PFS benefit was 7.2 versus 6.3 months between the Chinese study and the INVICTUS study, suggesting that ripretinib effectively delayed PD. The objective response rate (ORR) was 18.4% versus 11.8%, suggesting that ripretinib effectively shrank the tumors. The overall survival (OS) was not yet reached versus 18.2 months, suggesting that ripretinib offers significant survival benefits. In addition, ripretinib showed consistent safety profiles in Chinese and foreign populations (5).
Several TKIs have been approved to treat advanced GISTs. (I) Imatinib, for the treatment of Kit (CD117)-positive unresectable and/or metastatic malignant GISTs: according to the EORTC-62005 study, the median PFS after imatinib treatment was 18.9 months, the ORR was 51%, and the median OS was 55 months. Imatinib can be used for the postoperative adjuvant treatment of adult patients with Kit (CD117)-positive GISTs. (II) Sunitinib, for the treatment of GISTs after imatinib mesylate failure or intolerance. According to the STUDY1 study, the median PFS after sunitinib treatment was 5.6 months, the ORR was 6.8%, and the median OS was 17 months. (III) Regorafenib, for the treatment of locally advanced, unresectable, or metastatic GISTs previously treated with imatinib and sunitinib. According to the GRID study, the median PFS after regorafenib treatment was 4.8 months and the ORR was 4.5%. (IV) Ripretinib, for the treatment of advanced GISTs previously treated with three or more TKIs (including imatinib). According to the INVICTUS study, the median PFS after ripretinib treatment was 6.3 months, the ORR was 11.8%, and the median OS was 18.2 months. (V) Afatinib, for the treatment of unresectable or metastatic GISTs carrying a PDGFRA gene exon 18 mutation (including D842V). According to the NAVIGATOR study, the ORR after afatinib treatment was 84%.
In summary, the approval of novel drugs has brought new hope for the treatment of advanced GISTs. Clinical practice can accumulate more experience and better serve patients. As shown by Chinese and foreign studies, ripretinib has encouraging antitumor activity and a good safety profile in advanced GIST patients who have received three TKIs (including imatinib). Genotyping data from clinical research is also informative and valuable. For patients whose GIST harbors a primary KIT exon 11 mutation, a precise and individualized treatment protocol may be more beneficial.
Acknowledgments
Funding: This article is supported by “345 Talent Project”.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-534/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-534/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Terada T. Smooth muscles and stem cells of embryonic guts express KIT, PDGFRRA, CD34 and many other stem cell antigens: suggestion that GIST arise from smooth muscles and gut stem cells. Int J Clin Exp Pathol 2013;6:1038-45. [PubMed]
- Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018;24:2806-17. [Crossref] [PubMed]
- Jasek K, Buzalkova V, Minarik G, et al. Detection of mutations in the BRAF gene in patients with KIT and PDGFRA wild-type gastrointestinal stromal tumors. Virchows Arch 2017;470:29-36. [Crossref] [PubMed]
- Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019;35:738-751.e9. [Crossref] [PubMed]
- Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:923-34. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 2005;41:1751-7. [Crossref] [PubMed]
- Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64-74. [Crossref] [PubMed]
- Serrano C, Mariño-Enríquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer 2019;120:612-20. [Crossref] [PubMed]
- Blay JY, Kang YK, Nishida T, et al. Gastrointestinal stromal tumours. Nat Rev Dis Primers 2021;7:22. [Crossref] [PubMed]
- Reichardt P, Kang YK, Rutkowski P, et al. Clinical outcomes of patients with advanced gastrointestinal stromal tumors: safety and efficacy in a worldwide treatment-use trial of sunitinib. Cancer 2015;121:1405-13. [Crossref] [PubMed]
- Nannini M, Nigro MC, Vincenzi B, et al. Personalization of regorafenib treatment in metastatic gastrointestinal stromal tumours in real-life clinical practice. Ther Adv Med Oncol 2017;9:731-9. [Crossref] [PubMed]
- Bauer S, George S, von Mehren M, et al. Early and Next-Generation KIT/PDGFRA Kinase Inhibitors and the Future of Treatment for Advanced Gastrointestinal Stromal Tumor. Front Oncol 2021;11:672500. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


