
Association of social determinants of health with late diagnosis and survival of patients with pancreatic cancer
Introduction
Pancreatic adenocarcinoma (PC) is a lethal malignancy: predictions expect 60,430 new diagnoses of the disease in 2021 with 48,220 estimated deaths, and only 10% of patients are expected to live up until the 5-year mark (1). By 2030, PC is on track to be the second cause of malignancy-related deaths in the US (2). This disease does not impact all patients equally, as healthcare outcomes for patients with PC vary by race and ethnicity as well as socioeconomic factors.
Disparities in PC have been documented by race (3-5), age (6), sex (6), income (7,8), insurance (7,8), and location (9-11). More specifically, inequities exist in how patients are cared for and treated during the early stages of the disease. For example, it is known that Black and uninsured patients have lower rates of curative intent surgery (3,4,12-14). Additionally, Black patients and racial minorities are less likely to receive adjuvant chemotherapy (13,15-16). However, it is not fully understood what impact social determinants of health (SDH), such as income, education, race, and insurance status, have on the diagnosis of PC and survival of patients with Stage IV disease, for which no curative treatments exist. Moreover, one of the limitations of some previous studies is the lack of inclusion of education as a predictive factor (8,17,18). Given that some social risk characteristics are modifiable, the study of SDH could improve the early diagnosis rate and survival of patients with pancreatic cancer.
For this reason, our study aims to evaluate if health inequities in patients with PC are more prominent before or after the diagnosis of advanced disease. We hypothesize that SDH, such as income, education, race, and insurance status, are (I) associated with stage of diagnosis of PC (Stage IV vs. other stages) and (II) associated with overall survival (OS) in Stage IV patients.
In our study, we sought to answer two key questions. First, what factors are associated with late-stage (Stage IV) PC diagnoses? Specifically, are a patient’s race, insurance status, neighborhood income level, and/or neighborhood education level associated with late-stage diagnoses? Second, among those diagnosed with Stage IV PC, are these factors associated with survival? We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-788/rc).
Methods
Data source and patient population
The National Cancer Database (NCDB) was queried for this retrospective observational cohort study. The NCDB encompasses 70% of hospital-based cancers diagnosed in the US. NCDB data is deidentified. Eligibility included patients 18 years and older newly diagnosed with PC in the US between January 1, 2004 to December 31, 2016. We excluded cases with more than one cancer diagnosis and cases in which the treatment was administered at a facility different from the reporting one, as this might create bias. A complete case analysis method was used for handling missing data. Cases with missing data on predictors, outcomes, and confounders were eliminated (Figure S1). 230,877 cases were selected for the final analysis. The editions 6th and 7th of the American Joint Commission on Cancer (AJCC) editions were used, depending on year of diagnosis. AJCC analytic stage group is a variable in NCDB. It is assigned the value of the pathologic stage. Clinical stage is used if pathologic information was not available. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Outcomes
The primary outcome was late-stage diagnosis, Stage IV PC, vs. other stages (Stages 0-III). Staging variable was dichotomized for analysis (late or Stage IV vs. early or Stages 0-III). The secondary outcome was survival from time of diagnosis.
Predictor variables of interest
We used the following SDH as primary predictors: race, income, education, insurance. Race appears in the NCDB database as White, Black, and other individual twenty-seven races. These other individual race categories have fewer participants, and therefore, the race variable was defined in three categories: White, Black or other. The 2016 American Community Survey data was used to match the patient’s zip code with median household income and education. Data, spanned from 2012–2016, was inflation-adjusted. Four quartiles of income were used: <$40,227, $40,227–$50,353, $50,354–$63,332, and ≥$63,333. Four levels of high-school graduation rate were: <82.4%, 82.5–89.1%, 89.2–93.7%, or ≥93.7%.
Control variables
Multivariable models were adjusted for age, sex, and Charlson-Deyo comorbidity score. Age was included in the analysis in ordinal categories by deciles. Sex was included as a binary variable, male vs. female. The Charlson-Deyo comorbidity index is calculated from diagnosis codes, applying weight for type of comorbidity, then summed for a score from 0 to 25. Values were then collapsed into four categories: a score of 0, 1, 2, or greater than 3, with higher score representing more comorbidity.
Statistical analysis
Baseline characteristics for our cohort were first summarized by early vs. late-stage (Stage 0-III vs. Stage IV). A univariate logistic regression analysis was done to analyze the impact of each variable on a diagnosis of Stage IV PC (vs. Stages 0-III). We adjusted a multivariable logistic regression model for age, sex, Charlson comorbidity index (CCI), race, insurance, income, and education. A description of the models can be found in Table S1. Finally, we did univariate and multivariable Cox proportional hazard regression models to analyze predictors associated with survival of patients with Stage IV PC. Kaplan-Meier curves were generated to illustrate differences in survival times between the categories of each of the four social determinants. Statistical analyses were done with Stata IC/SE version 16 (College Station, TX, USA). Significance was defined by P≤0.05.
Sensitivity analysis
We repeated the multivariable Cox proportional hazard regression model to document predictors associated with survival of PC patients of all stages.
Results
Patient characteristics
230,877 patients were included for the final statistical analysis. The median age was 68 years with a mean of 67.3 (standard deviation 12.1). There were 113,881 (49%) female patients and 116,996 (51%) male patients included in the sample. Stage IV population encompassed 114,106 of the patients or a total of 49%. Only a small percentage of the included individuals lacked insurance (3%, 7,828), whereas the majority (55%, 127,094) had Medicare or private insurance (34%, 79,212). The majority of the patients were White (192,338), vs. Black (29,453). There was a relatively homogenous distribution of educational attainment across the four quartiles. Additional demographic characteristics can be found in Table 1.
Table 1
| Characteristics at diagnosis | Total, N=230,877 | Stage 0–III, N=116,771 | Stage IV, N=114,106 |
|---|---|---|---|
| Age, deciles | |||
| 18–19 | 53 (0%) | 45 (0%) | 8 (0%) |
| 20–29 | 651 (0%) | 435 (0%) | 216 (0%) |
| 30–39 | 2,623 (1%) | 1,419 (1%) | 1,204 (1%) |
| 40–49 | 13,703 (6%) | 6,888 (6%) | 6,815 (6%) |
| 50–59 | 43,770 (19%) | 21,645 (19%) | 22,125 (19%) |
| 60–69 | 67,562 (29%) | 34,214 (29%) | 33,348 (29%) |
| 70–79 | 62,360 (27%) | 32,179 (28%) | 30,181 (26%) |
| 80–89 | 34,929 (15%) | 17,432 (15%) | 17,497 (15%) |
| ≥90 | 5,226 (2%) | 2,514 (2%) | 2,712 (2%) |
| Sex | |||
| Female | 113,881 (49%) | 59,486 (51%) | 54,395 (48%) |
| Male | 116,996 (51%) | 57,285 (49%) | 59,711 (52%) |
| Race | |||
| White | 192,338 (83%) | 97,834 (84%) | 94,504 (83%) |
| African American | 29,453 (13%) | 14,203 (12%) | 15,250 (13%) |
| Other | 9,086 (4%) | 4,734 (4%) | 4,352 (4%) |
| Percent high school attainmenta | |||
| <82.4 | 48,984 (21%) | 24,331 (21%) | 24,653 (22%) |
| 82.5–89.1 | 60,577 (26%) | 30,384 (26%) | 30,193 (26%) |
| 89.2–93.7 | 65,464 (28%) | 33,313 (29%) | 32,151 (28%) |
| >93.7 | 55,852 (24%) | 28,743 (25%) | 27,109 (24%) |
| Annual household incomeb | |||
| <$40,227 | 44,704 (19%) | 22,165 (19%) | 22,539 (20%) |
| $40,227–$50,353 | 51,215 (22%) | 25,953 (22%) | 25,262 (22%) |
| $50,354–$63,332 | 53,598 (23%) | 27,106 (23%) | 26,492 (23%) |
| ≥$63,333 | 81,360 (35%) | 41,547 (36%) | 39,813 (35%) |
| Insurance status | |||
| No insurance | 7,828 (3%) | 3,332 (3%) | 4,496 (4%) |
| Private | 79,212 (34%) | 40,862 (35%) | 38,350 (34%) |
| Medicaid | 14,118 (6%) | 6,475 (6%) | 7,643 (7%) |
| Medicare | 127,094 (55%) | 64,634 (55%) | 62,460 (55%) |
| Other government | 2,625 (1%) | 1,468 (1%) | 1,157 (1%) |
| Charlson Comorbidity indexc | |||
| 0 | 150,729 (65%) | 76,664 (66%) | 74,065 (65%) |
| 1 | 57,205 (25%) | 29,243 (25%) | 27,962 (25%) |
| 2 | 14,878 (6%) | 7,396 (6%) | 7,482 (7%) |
| ≥3 | 8,065 (3%) | 3,468 (3%) | 4,597 (4%) |
a, Percentage with high-school education from ACS 2016 matched with patient’s zip code. b, In US dollars–Median household income from ACS 2016 matched with patient’s zip-code. c, Charlson-Deyo Comorbidity score. ACS, American Community Survey.
Factors associated with late-stage diagnosis
In univariate analysis, education [>93% high school completion (HSC) vs. <82.4%, OR 0.93 (0.91–0.95)], income [>$63,333 vs. <$40,277, OR 0.94 (0.92–0.96)], and insurance [private vs. no, OR 0.70 (0.66–0.73)] significantly decreased the odds of Stage IV PC. Black race was associated with higher odds of Stage IV PC [vs. White, OR 1.11 (1.08–1.14)] (Table 2; Figure 1). In the multivariable analysis, education [>93% HSC vs. <82.4%, OR 0.96 (0.93–0.99)] and having insurance [private vs. no, OR 0.72 (0.67–0.74)] significantly decreased the risk of a late diagnosis, whereas Black race increased the odds of a late diagnosis [vs. White, OR 1.09 (1.07–1.12)].
Table 2
| AJCC Stage IV vs. Stages 0–III | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age (deciles) | 1.00 (1.00–1.01) | 0.154 | 1.03 (1.02–1.04) | <0.001 | |
| Race (vs. White) | |||||
| Black | 1.11 (1.08–1.14) | <0.001 | 1.09 (1.07–1.12) | <0.001 | |
| Other | 0.95 (0.91–0.99) | 0.021 | 0.93 (0.90–0.98) | 0.002 | |
| Sex (vs. female) | |||||
| Male | 1.14 (1.12–1.16) | <0.001 | 1.15 (1.13–1.17) | <0.001 | |
| Percent high school attainmenta | |||||
| 82.5–89.1 | 0.98 (0.96–1.00) | 0.109 | 1.00 (0.98–1.03) | 0.981 | |
| 89.2–93.7 | 0.95 (0.93–0.98) | <0.001 | 0.98 (0.95–1.01) | 0.146 | |
| >93.7 | 0.93 (0.91–0.95) | <0.001 | 0.96 (0.93–0.99) | 0.011 | |
| Income (vs. <$40,227)b | |||||
| $40,227–$50,353 | 0.96 (0.93–0.98) | 0.001 | 0.99 (0.96–1.02) | 0.45 | |
| $50,354–$63,332 | 0.96 (0.94–0.99) | 0.002 | 1.01 (0.98–1.04) | 0.503 | |
| >$63,333 | 0.94 (0.92–0.96) | <0.001 | 1.01 (0.98–1.04) | 0.407 | |
| Insurance status (vs. no insurance) | |||||
| Private | 0.70 (0.66–0.73) | <0.001 | 0.72 (0.67–0.74) | <0.001 | |
| Medicaid | 0.87 (0.83–0.92) | <0.001 | 0.87 (0.83–0.92) | <0.001 | |
| Medicare | 0.72 (0.68–0.75) | <0.001 | 0.70 (0.66–0.73) | <0.001 | |
| Other government | 0.58 (0.53–0.64) | <0.001 | 0.57 (0.52–0.63) | <0.001 | |
| Charlson Comorbidity index (vs. 0 score)c | |||||
| 1 | 0.99 (0.97–1.01) | 0.294 | 0.98 (0.96–1.00) | 0.054 | |
| 2 | 1.05 (1.01–1.08) | 0.007 | 1.03 (1.00–1.07) | 0.04 | |
| ≥3 | 1.27 (1.31–1.43) | <0.001 | 1.34 (1.28–1.40) | <0.001 | |
a, percentage with high-school education from ACS 2016 matched with patient’s zip code. b, in US dollars–Median household income from ACS 2016 matched with patient’s zip-code. c, Charlson-Deyo Comorbidity score. AJCC, American Joint Committee on Cancer; ACS, American Community Survey.
Factors associated with overall survival
In univariate Cox analysis, higher income [>$63,333 (vs. <$40,277), HR 0.82 (0.81–0.83)], insurance [private vs. no, HR 0.77 (0.73–0.76)] and having more education (>93% HSC vs. <82.4%, HR 0.87 (0.86–0.88)] improved OS. Black race was associated with lower OS [vs. White, HR 1.03 (1.02–1.05)] (Table 3). In the multivariable Cox analysis, only higher income [>$63,333 (vs. <$40,277), HR 0.87 (0.85–0.89)] and having insurance [private vs. no, HR 0.77 (0.74–0.79)] were associated with improved OS, while Black race did not impact survival [vs. White, OR 1.00 (0.98–1.01)] (Figures 2,3). Older age, male sex, and a higher CCI were associated with worse survival in univariate and multivariable analysis.
Table 3
| Survival | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age (deciles) | 1.27 (1.26–1.27) | <0.001 | 1.25 (1.24–1.25) | <0.001 | |
| Race (vs. White) | |||||
| Black | 1.03 (1.02–1.05) | <0.001 | 1.00 (0.98–1.01) | 0.952 | |
| Other | 0.86 (0.84–0.88) | <0.001 | 0.90 (0.87–0.93) | <0.001 | |
| Sex (vs. female) | |||||
| Male | 1.02 (1.01–1.02) | <0.001 | 1.05 (1.03–1.06) | <0.001 | |
| Percent high school attainmenta | |||||
| 82.5–89.1 | 0.99 (0.98–1.00) | 0.074 | 1.05 (1.03–1.07) | <0.001 | |
| 89.2–93.7 | 0.94 (0.93–0.95) | <0.001 | 1.04 (1.01–1.06) | <0.001 | |
| >93.7 | 0.87 (0.86–0.88) | <0.001 | 0.99 (0.97–1.01) | 0.543 | |
| Income (vs. <$40,227)a,b | |||||
| $40,227–$50,353 | 0.94 (0.93–0.96) | <0.001 | 0.96 (0.94–0.97) | <0.001 | |
| $50,354–$63,332 | 0.90 (0.89–0.91) | <0.001 | 0.92 (0.90–0.94) | <0.001 | |
| >$63,333 | 0.82 (0.81–0.83) | <0.001 | 0.87 (0.85–0.89) | <0.001 | |
| Insurance status (vs. no insurance) | |||||
| Private | 0.74 (0.73–0.76) | <0.001 | 0.77 (0.74–0.79) | <0.001 | |
| Medicaid | 0.94 (0.92–0.97) | <0.001 | 0.93 (0.89–0.96) | <0.001 | |
| Medicare | 1.11 (1.09–1.15) | <0.001 | 0.84 (0.82–0.87) | <0.001 | |
| Other government | 0.85 (0.81–0.89) | <0.001 | 0.84 (0.79–0.90) | <0.001 | |
| Charlson score (vs. 0 score)c | |||||
| 1 | 1.12 (1.11–1.13) | <0.001 | 1.12 (1.16–1.14) | <0.001 | |
| 2 | 1.34 (1.32–1.37) | <0.001 | 1.32 (1.28–1.35) | <0.001 | |
| ≥3 | 1.69 (1.65–1.73) | <0.001 | 1.68 (1.63–1.73) | <0.001 | |
a, percentage with high-school education from ACS 2016 matched with patient’s zip code. b, in US dollars–Median household income from ACS 2016 matched with patient’s zip-code. c, Charlson-Deyo Comorbidity score. ACS, American Community Survey.
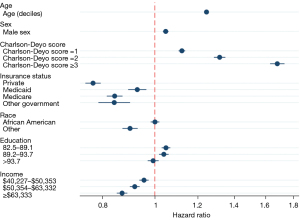

In a sensitivity analysis, we evaluated the association of SDHs in the survival of patients with any stage of PC. Education, income, and insurance were associated with improved survival. Race was not significantly associated with survival (Table S2).
Discussion
Our study showed that patients diagnosed with Stage IV PC were more likely to have lower education levels, be without insurance and be Black. Once diagnosed with metastatic disease, patients without insurance and those living in lower-income neighborhoods had shorter survival. Race and education did not have a significant impact on survival for those with Stage IV PC. These findings suggest that disparities in rates of late-stage diagnosis differ meaningfully from disparities in cancer survival. The absence of insurance was the most important factor along the continuum of PC care, from diagnosis to treatment.
Prior literature explored disparities in PC outcomes, finding that race, ethnicity, geographical location, and other socioeconomic factors impact PC survival (5,19), with unequal treatments driving much of these disparities (3,7,14,16,17). Our research builds upon this prior work by exploring the relationship between socioeconomic factors and diagnosis and survival of patients with PC with a specific focus on outcomes in the palliative setting. Furthermore, it delineates which socioeconomic factors are associated with a Stage IV diagnosis and which ones are associated with improved survival.
To our knowledge, this is the first study to show that education is an impactful factor in earlier diagnosis of PC. It is possible that more educated patients readily recognize concerning symptoms of PC such as weight loss, early satiety, and abdominal pain. These findings put an onus on public health policymakers to evaluate education tools in the community and develop strategies for disseminating knowledge about pancreatic malignancy signs and symptoms. Alternatively, higher education may be a surrogate for access to resources which facilitate follow-up on symptoms, risk factors or family history. While education was associated with early diagnosis, there were no differences in survival according to education for patients with metastatic disease. This suggests that despite the importance of any knowledge about the condition, once the disease is incurable, survival outcomes are superseded by other factors and resources, such as insurance and income.
Prior studies have shown that race is an independent predictor of a late diagnosis of PC and poor survival in all stages (20-24). In these studies, the survival differences by race were driven largely by differences in surgery and treatment rates in early stage or potentially curative disease settings (3,4,12,25). However, our analyses were instead focused on those with metastatic disease, eliminating the administration of curative intent treatments as mediators. Our study demonstrates that there is no impact of race on survival in the metastatic disease setting, suggesting that the racial disparities in PC survival in prior studies may be partially due to treatment differences and access to care in early and potentially curative stages. Race was also not associated with differences in survival in a study done in an integrated healthcare system, when patients with PC had equal access to curative intent and palliative interventions (26).
Finally, the present study's finding that uninsured patients are most likely to have stage IV at diagnosis and have worse survival is compatible with results by other investigators (7,15,27). Some studies have shown that improving insurance coverage can mitigate disparities in PC treatment, at least at the state level, as this can increase access to curative treatment (28). Our research builds upon prior research by demonstrating the importance of insurance coverage for those with late-stage PC across the care continuum, from initial diagnosis to treatment.
The current study results demonstrated that poor education, Black race, and being uninsured are predictors of a late diagnosis of PC. Once diagnosed and being treated with palliative goals, the two most important socioeconomic predictors of survival are income and insurance. Given that PC is expected to be the second cause of cancer-associated deaths by the end of this decade, these findings have important implications for public health policy. Our study suggests that education is an important factor at diagnosis. This is an argument in favor of community-level educational interventions. It shines a light on the importance of disseminating knowledge about symptoms of PC. For example, a person can easily dismiss abdominal pain, depending on the degree or chronicity. Similarly, new onset adult diabetes can also herald an underlying pancreatic pathology. Perhaps if we emphasized these points, patients and caregivers might be more empowered to seek attention sooner.
Regardless of education and empowerment, our study demonstrated that insurance coverage is the most important factor in the diagnosis and survival of patients with PC, suggesting the importance of expanding access to care through regional or national health policies. Reducing disparities in cancer care will require the collective effort of many key stakeholders, including policymakers, public health officials, and healthcare providers.
Our study has several limitations. First, though the dataset was from accredited CoC institutions, there is the risk of misclassification bias when using the dataset from the NCDB. Furthermore, because the NCDB is a hospital-based database, the generalizability of the study may be limited and subject to some degree due to regionalization and by selection bias whereby patients needed to access a hospital in order to be captured by the NCDB. While this may introduce some limitations in our patient sample, it is unlikely to influence our results substantially, as the NCDB includes 70% of the US’s hospital-based cancer population with representation by all states and Puerto Rico. Second, some of the variables, such as income and education, do not represent granular data. Instead, they are ecologic variables, assigned according to the patient’s primary residence zip code, matched to the American Community Survey. Although this precludes the assignment of individual causation, it gives us an approximate idea of the importance of the different socioeconomic factors. Third, when analyzing race, there is some residual confounding in the analysis of patients of Black race. For example, some of the Black patients included in this study were of Hispanic descent, and others were not. Hispanics have a heterogeneous ancestry, including Indigenous, Caucasian, and African ancestry. Since other studies have evaluated this ethnic group (24,29), we chose to focus on the differences between Black vs. White patients irrespective of ethnicity, as we believe this would partially eliminate biases from heterogeneous ancestry. We acknowledge this is not the generally accepted standard evaluation of race/ethnicity and that even black ancestry has significant heterogeneity. Fourth, to handle missing variables, we used complete case analysis under the assumption that variables were missing at random. While this may not be an accurate assumption, complete case analysis is an accepted statistical technique to handle missing data.
Conclusions
The late diagnosis of PC is a death sentence for most patients with an increasing burden on the healthcare system and communities. Our study adds to the collective body of knowledge relating key SDH that impact the continuum of care for patients with advanced pancreatic cancer, including stage at diagnosis and overall survival.
Acknowledgments
The authors gratefully acknowledge Yvonne Mullowney and Billings Clinic Collaborative Science & Innovation for assistance in technical editing and proofreading the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-788/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-788/coif). JMB discloses serving on the Speakers’ Bureau for Daiichi Sankyo and Aztra-Zeneca. JCF discloses institutional research support from: BMS, Merck, Astra Zeneca, Likely, Bayer, Ipsen, GSK, Astellas Pharma, Genentech, and BioMed Valley Discoveries. TJG discloses consulting fees from Tempus and Pfizer, and Research support (Institution): BMS, Merck, Astra Zeneca, Likely, Bayer, Ipsten, GSK, Astellas Pharma, Genetech, BioMed Valley Discoveries. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The NCDB file was provided to the authors for analytical purposes and was exempt from institutional review board review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Bliton JN, Parides M, Muscarella P, et al. Understanding Racial Disparities in Gastrointestinal Cancer Outcomes: Lack of Surgery Contributes to Lower Survival in African American Patients. Cancer Epidemiol Biomarkers Prev 2021;30:529-38. [Crossref] [PubMed]
- Blanco BA, Poulson M, Kenzik KM, et al. The Impact of Residential Segregation on Pancreatic Cancer Diagnosis, Treatment, and Mortality. Ann Surg Oncol 2021;28:3147-55. [Crossref] [PubMed]
- Cervantes A, Waymouth EK, Petrov MS. African-Americans and Indigenous Peoples Have Increased Burden of Diseases of the Exocrine Pancreas: A Systematic Review and Meta-Analysis. Dig Dis Sci 2019;64:249-61. [Crossref] [PubMed]
- Azap RA, Hyer JM, Diaz A, et al. Sex-based differences in time to surgical care among pancreatic cancer patients: A national study of Medicare beneficiaries. J Surg Oncol 2021;123:236-44. [Crossref] [PubMed]
- Moaven O, Richman JS, Reddy S, et al. Healthcare disparities in outcomes of patients with resectable pancreatic cancer. Am J Surg 2019;217:725-31. [Crossref] [PubMed]
- Mehta VV, Friedmann P, McAuliffe JC, et al. Pancreatic Cancer Surgery Following Emergency Department Admission: Understanding Poor Outcomes and Disparities in Care. J Gastrointest Surg 2021;25:1261-70. [Crossref] [PubMed]
- Yee EK, Coburn NG, Davis LE, et al. Impact of Geography on Care Delivery and Survival for Noncurable Pancreatic Adenocarcinoma: A Population-Based Analysis. J Natl Compr Canc Netw 2020;18:1642-50. [Crossref] [PubMed]
- Sheikh M, Masoudi S, Bakhshandeh R, et al. Survival features, prognostic factors, and determinants of diagnosis and treatment among Iranian patients with pancreatic cancer, a prospective study. PLoS One 2020;15:e0243511. [Crossref] [PubMed]
- Siegel J, Engelhardt KE, Hornor MA, et al. Travel distance and its interaction with patient and hospital factors in pancreas cancer care. Am J Surg 2021;221:819-25. [Crossref] [PubMed]
- Molina G, Clancy TE, Tsai TC, et al. Racial Disparity in Pancreatoduodenectomy for Borderline Resectable Pancreatic Adenocarcinoma. Ann Surg Oncol 2021;28:1088-96. [Crossref] [PubMed]
- Swords DS, Mulvihill SJ, Brooke BS, et al. Disparities in utilization of treatment for clinical stage I-II pancreatic adenocarcinoma by area socioeconomic status and race/ethnicity. Surgery 2019;165:751-9. [Crossref] [PubMed]
- Tohme S, Kaltenmeier C, Bou-Samra P, et al. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann Surg Oncol 2018;25:3427-35. [Crossref] [PubMed]
- Cloyd JM, Shen C, Santry H, et al. Disparities in the Use of Neoadjuvant Therapy for Resectable Pancreatic Ductal Adenocarcinoma. J Natl Compr Canc Netw 2020;18:556-63. [Crossref] [PubMed]
- Sanford NN, Aguilera TA, Folkert MR, et al. Sociodemographic Disparities in the Receipt of Adjuvant Chemotherapy Among Patients With Resected Stage I-III Pancreatic Adenocarcinoma. J Natl Compr Canc Netw 2019;17:1292-300. [Crossref] [PubMed]
- Nipp R, Tramontano AC, Kong CY, et al. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med 2018;7:525-35. [Crossref] [PubMed]
- Tavakkoli A, Singal AG, Waljee AK, et al. Racial Disparities and Trends in Pancreatic Cancer Incidence and Mortality in the United States. Clin Gastroenterol Hepatol 2020;18:171-178.e10. [Crossref] [PubMed]
- Lutfi W, Zenati MS, Zureikat AH, et al. Health Disparities Impact Expected Treatment of Pancreatic Ductal Adenocarcinoma Nationally. Ann Surg Oncol 2018;25:1860-7. [Crossref] [PubMed]
- Greenbaum A, Alkhalili E, Rodriguez R, et al. Pancreatic Adenocarcinoma in New Mexico Native Americans: Disparities in Treatment and Survival. J Health Care Poor Underserved 2019;30:609-17. [Crossref] [PubMed]
- Gad MM, Saad AM, Al-Husseini MJ, et al. Temporal trends of pancreatic ductal adenocarcinoma in young adults in the United States: A Population-Based Study. Clin Res Hepatol Gastroenterol 2020;44:204-10. [Crossref] [PubMed]
- Fagenson AM, Grossi SM, Musgrove K, et al. Ethnic and racial disparities of pancreatic adenocarcinoma in Florida. HPB (Oxford) 2020;22:735-43. [Crossref] [PubMed]
- Noel M, Fiscella K. Disparities in Pancreatic Cancer Treatment and Outcomes. Health Equity 2019;3:532-40. [Crossref] [PubMed]
- Lara LF, Bellin MD, Ugbarugba E, et al. A Study on the Effect of Patient Characteristics, Geographical Utilization, and Patient Outcomes for Total Pancreatectomy Alone and Total Pancreatectomy With Islet Autotransplantation in Patients With Pancreatitis in the United States. Pancreas 2019;48:1204-11. [Crossref] [PubMed]
- Thobie A, Mulliri A, Bouvier V, et al. Same Chance of Accessing Resection? Impact of Socioeconomic Status on Resection Rates Among Patients with Pancreatic Adenocarcinoma-A Systematic Review. Health Equity 2021;5:143-50. [Crossref] [PubMed]
- Lee S, Reha JL, Tzeng CW, et al. Race does not impact pancreatic cancer treatment and survival in an equal access federal health care system. Ann Surg Oncol 2013;20:4073-9. [Crossref] [PubMed]
- Swords DS, Mulvihill SJ, Brooke BS, et al. Size and Importance of Socioeconomic Status-Based Disparities in Use of Surgery in Nonadvanced Stage Gastrointestinal Cancers. Ann Surg Oncol 2020;27:333-41. [Crossref] [PubMed]
- Loehrer AP, Chang DC, Hutter MM, et al. Health Insurance Expansion and Treatment of Pancreatic Cancer: Does Increased Access Lead to Improved Care? J Am Coll Surg 2015;221:1015-22. [Crossref] [PubMed]
- Gabriel E, Thirunavukarasu P, Attwood K, et al. National disparities in minimally invasive surgery for pancreatic tumors. Surg Endosc 2017;31:398-409. [Crossref] [PubMed]



