
The efficacy and safety of gemcitabine-based combination therapy vs. gemcitabine alone for the treatment of advanced pancreatic cancer: a systematic review and meta-analysis
Introduction
Pancreatic cancer is a malignant tumor with high mortality. Even with continued improvements in diagnostic technology, most patients with pancreatic cancer are often diagnosed at an unresectable, advanced stage (1,2). Currently, surgical resection is the only possible cure, but patients with advanced pancreatic cancer have usually missed their chance of surgery. The prognosis of patients with pancreatic cancer is often poor, the median survival time is only 3–6 months for patients with distant metastasis, and that of patients with local complications is only 6–10 months (3,4). Therefore, it is urgent to seek an effective chemotherapy regimen to improve the prognosis of pancreatic cancer.
Gemcitabine (GEM), a synthetic analog of cytarabine, whose structure is similar to that of deoxycytidine and cytarabine, is one of the most used chemotherapeutic drugs for pancreatic cancer (5,6). In 1997, GEM-based chemotherapy was first proposed as a standard therapy treatment for patients with unresectable pancreatic cancer. In recent decades, GEM has become a standard drug for chemotherapy of pancreatic cancer and a critical target drug for research (7,8). Most patients with advanced pancreatic cancer have symptoms such as severe pain, jaundice, weight loss, nausea, vomiting, and general weakness. Although the short-term objective efficacy of GEM for advanced pancreatic cancer is not obvious and the complete or partial remission rate is not high, at only 10–30%, research has found that GEM has a significant effect on the clinical benefit rate (CBR), such as the degree of pain, the dosage of painkillers, and weight gain, which greatly enhances the quality of life of patients with pancreatic cancer (9,10).
At the same time, in order to improve the therapeutic effect of pancreatic cancer treatment, since GEM entered the market, researchers have been trying to treat advanced pancreatic cancer based on GEM combined with many drugs, which has significantly improved the overall survival (OS) and progression-free survival (PFS) of patients (11-13). In particular, there have been many studies conducted on the combination of GEM with platinum- or fluorouracil-based drugs. Cisplatin (CIS) is the main platinum-based drug, and fluorouracil-based drugs include capecitabine (CAP) and S-1 (14,15). These 3 drugs have been shown to be effective in the treatment of pancreatic cancer. However, some studies have also shown that although the combined chemotherapy yielded a significant improvement in the overall response rate (ORR), some trials did not show a significant extension of OS (16,17). Some studies have also shown that 1-year survival rate, the median survival time, and CBR of combined chemotherapy were low, and the clinical treatment effect was not very satisfactory (18,19).
Most clinical randomized controlled trials (RCTs) have shown that GEM-based combination therapy is better than single drug treatment, while some RCTs have drawn the opposite conclusions (20-22). Therefore, there is no clear certainty whether GEM-based combination therapy is better than single drug chemotherapy. This article collects the efficacy and safety outcomes of GEM-based combination therapy vs. GEM alone for advanced pancreatic cancer, and conducted a systematic review and meta-analysis, in order to provide some guidance for clinical chemotherapy of advanced pancreatic cancer. We present the following article in accordance with the PRISMA reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-624/rc).
Methods
Literature search strategy
Eight databases were carefully searched from their inception to 1 April 2022 without limitations of language and publication status: PubMed, Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese BioMedical Database (CBM), China Scientific Journal Database (VIP), and Wanfang Database. The following keywords were applied to search literature in combination with the Boolean operators ‘AND’ or ‘OR’: “gemcitabine”, “capecitabine”, “S-1”, “cisplatin”, and “pancreatic cancer”. At present, GEM plus CAP, GEM plus S-1 and GEM plus CIS were the main combination therapies. To identify additional eligible studies, we reviewed reference lists from eligible trials and relevant reviews and guidelines. Any disagreements in the first or second phases were determined by discussion and consensus between the two reviewers.
Study selection
The inclusion criteria of the selected literatures were as follows: (I) patients with advanced pancreatic cancer; (II) research comparing patients receive GEM-based combination therapy vs. GEM alone; (III) studies were designed as RCT; (IV) researches on comparison of the efficacy and safety outcomes, such as OS, PFS, ORR, 1-year survival rate, and adverse events (AEs).
Data extraction
We designed a data extraction form by consensus. One of the researchers performed all of the data extraction, and two investigators conducted independent verification. All of the above procedure were completed by two authors independently (Z Zhang, Shu He). The following contents were extracted from each article: first author’s name, year of publication, study design, country, trial phase, intervention group, sample size, participant characteristics (gender, age), and the results of some outcome variables.
Quality assessment
The Cochrane Collaboration’s risk of bias tool (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials) was used to assess the methodological quality of the selected studies, based on the following 7 items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. The total score of quality assessment was 8 points, which are scored by our two authors respectively. In case of disagreement, the final score was decided by the third author. We defined the score of 6–8 points as low risk (‘good’ quality), 3–5 points as unclear risk (‘moderate’ quality), and 0–2 points as high risk (‘poor’ quality).
Statistical analysis
Meta-analysis was conducted using the software Review Manager (RevMan 5.4, the Nordic Cochrane Centre, Copenhagen, Denmark). The outcome variables of this study included survival variables and dichotomous variables. The combined analysis of survival variables was presented by hazard ratio (HR) with 95% confidence interval (CI), and the pooled analysis of dichotomous variables was expressed by odds ratio (OR) with 95% CI. The Cochran Q test and the Higgins I-squared (I2) statistic were used for heterogeneity testing. When I2>50% or P<0.10, a random effects model was used, otherwise a fixed effects model was used. We conducted the subgroup analysis according to the added drugs of combination therapy. The funnel plot and Egger’s test were performed to detect the publication bias. P value was used to detect the statistical difference, which was statistically significant when P<0.05.
Results
Search process
A total of 1,678 potentially unique studies were identified. After removal of duplicates, a total of 1,465 records were remained. By reading the titles and abstracts, an additional 1,251 records were further excluded. Then, 197 articles were further excluded because of different study design or insufficient data available. A total of 17 studies were included in the final meta-analysis (20-36). The results of the search process were illustrated in a flowchart (Figure 1).
Characteristics of included studies
Table 1 listed the chief characteristics of the 17 selected trials. All the trials included were phase II or III. All of the control groups were GEM alone. In the intervention groups, 7 were the combination of GEM and CAP, 5 were the combination of GEM and S-1, and 5 were the combination of GEM and CIS. Totals of 2,370 and 2,827 patients were included in the test group and control group, respectively. The age of participants ranged from 27 to 85 years. The median time of OS and PFS in the test group and the control group of each article were shown in Table 1, where it can be seen that the median time of OS and PFS in the test group is greater than that in the control group.
Table 1
| Study | Country | Study design | Phase | Intervention | No. of patients | Gender (M/F) | Age (years), median [range] | OS (months) | PFS (months) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Control | Test | Control | Test | Control | Test | Control | Test | Control | Test | Control | |||||||||
| Scheithauer, 2003 | Austria | RCT | II | GEM + CAP | GEM | 41 | 42 | 27/14 | 23/19 | 64 [40–75] | 66 [39–75] | 9.5 | 8.2 | 5.1 | 4.0 | |||||
| Herrmann, 2007 | Germany | RCT | III | GEM + CAP | GEM | 160 | 159 | 86/74 | 85/74 | 62 [27–83] | 62 [36–84] | 8.4 | 7.2 | 4.3 | 3.9 | |||||
| Bernhard, 2008 | Germany | RCT | III | GEM + CAP | GEM | 160 | 159 | 86/74 | 85/74 | 62 [27–83] | 62 [36–84] | – | – | – | – | |||||
| Cunningham, 2009 | UK | RCT | III | GEM + CAP | GEM | 267 | 266 | 160/107 | 153/113 | 62 [37–82] | 62 [26–83] | 7.1 | 6.2 | 5.3 | 3.8 | |||||
| Lee, 2017 | Korea | RCT | III | GEM + CAP | GEM | 108 | 106 | 63/45 | 57/49 | 64 [37–80] | 64 [43–85] | 10.3 | 7.5 | 6.2 | 5.3 | |||||
| Neoptolemos, 2017 | UK | RCT | III | GEM + CAP | GEM | 364 | 366 | 202/162 | 212/154 | 65 [39–81] | 65 [37–80] | 28.0 | 25.5 | – | – | |||||
| de Jong, 2022 | Netherlands | RCT | III | GEM + CAP | GEM | 164 | 614 | 78/86 | 342/272 | 66 [58–71] | 67 [60–72] | 31.4 | 22.1 | – | – | |||||
| Nakai, 2012 | Japan | RCT | II | GEM + S-1 | GEM | 53 | 53 | 42/11 | 33/20 | 63 [40–82] | 67 [42–84] | 13.5 | 8.8 | 5.4 | 3.6 | |||||
| Ozaka, 2012 | Japan | RCT | II | GEM + S-1 | GEM | 53 | 59 | 32/21 | 35/24 | 64 [45–77] | 64 [41–79] | 13.7 | 8.0 | 6.15 | 3.78 | |||||
| Sudo, 2013 | Japan | RCT | II | GEM + S-1 | GEM | 51 | 50 | 27/24 | 34/16 | 66 [50–77] | 67 [45–73] | 8.6 | 8.6 | 5.3 | 3.8 | |||||
| Ueno, 2013 | Japan | RCT | III | GEM + S-1 | GEM | 275 | 277 | 158/117 | 170/107 | – | – | 10.1 | 8.8 | 5.7 | 4.1 | |||||
| Imaoka, 2016 | Japan | RCT | III | GEM + S-1 | GEM | 275 | 277 | 158/117 | 170/107 | – | – | 10.2 | 8.5 | 6.9 | 4.5 | |||||
| Colucci, 2002 | Italy | RCT | III | GEM + CIS | GEM | 53 | 54 | 35/18 | 27/27 | 60 [33–71] | 63 [43–75] | 2.5 | 1.7 | 1.7 | 0.7 | |||||
| Heinemann, 2006 | Germany | RCT | III | GEM + CIS | GEM | 98 | 97 | 64/34 | 60/37 | 64 [37–82] | 66 [43–85] | 7.5 | 6.0 | 5.3 | 3.1 | |||||
| Palmer, 2007 | UK | RCT | II | GEM + CIS | GEM | 26 | 24 | 13/13 | 13/11 | 66 [40–79] | 66 [47–78] | 15.6 | 9.9 | – | – | |||||
| Colucci, 2010 | Italy | RCT | III | GEM + CIS | GEM | 201 | 199 | 125/76 | 113/86 | 63 [35–75] | 63 [37–75] | 7.2 | 8.3 | 3.8 | 3.9 | |||||
| Chao, 2013 | China | RCT | II | GEM + CIS | GEM | 21 | 25 | 17/4 | 18/7 | 69 [47–81] | 69 [46–83] | 7.9 | 7.7 | 3.6 | 4.6 | |||||
RCT, randomized controlled trial; GEM, gemcitabine; CAP, capecitabine; CIS, cisplatin; OS, overall survival; PFS, progression-free survival.
Results of quality assessment
The quality of the selected studies were assessed in accordance with the Cochrane tool for risk of bias. Among the included studies, high risk of performance bias was detected in 7 articles and detection bias was found in 3 studies (Figure 2). Figure 3 summarized the risk of bias for each included study.
Results of meta-analysis
OS
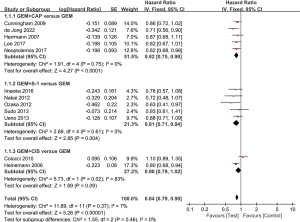
A total of 11 studies reported OS. The results of heterogeneity testing showed that there was insignificant heterogeneity among the included literature (I2=7%; P=0.37), so the combined effect quantity was analyzed by the fixed effects model. The overall meta-analysis showed that HR =0.84 (95% CI: 0.79 to 0.90; P<0.00001), suggesting that GEM-based combination therapy can effectively improve OS (Figure 4). Subgroup analysis was carried out according to the added drugs and they were divided into a GEM plus CAP group, GEM plus S-1 group, and GEM plus CIS group. The subgroup analysis results showed that GEM plus CAP group and GEM plus S-1 group could effectively improve OS, and the HR values were 0.82 (95% CI: 0.75 to 0.90; P<0.0001), and 0.81 (95% CI: 0.71 to 0.94; P=0.004), respectively. Compared with GEM alone, GEM plus CIS group did not improve OS, and its HR was: 0.90 (95% CI: 0.79 to 1.02; P=0.09).
PFS
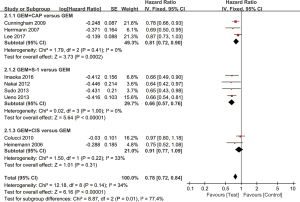
A total of 9 studies had data available for analysis of PFS. No significant heterogeneity was found among the included literature (I2=34%; P=0.14). The overall meta-analysis results showed that HR was 0.78 (95% CI: 0.72 to 0.84; P<0.00001), indicating that GEM-based combination therapy can effectively improve PFS (Figure 5). The results of subgroup analysis showed that the GEM plus CAP group (HR =0.81, 95% CI: 0.72 to 0.90; P=0.0002) and the GEM plus S-1 group (HR =0.66, 95% CI: 0.57 to 0.76; P<0.00001) could effectively improve PFS, but GEM plus CIS group did not achieve an improvement, and its HR was 0.91 (95% CI: 0.77 to 1.09; P=0.31).
ORR
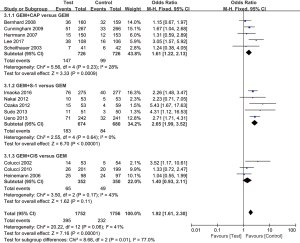
Thirteen trials evaluated ORR between GEM-based combination therapy and GEM alone. We used a fixed effect model as the moderate heterogeneity among the included literature (I2=41%; P=0.06). The overall meta-analysis results showed that GEM-based combination therapy can effectively increase ORR (OR =1.92; 95% CI: 1.61 to 2.30; P<0.00001) (Figure 6). Subgroup analysis also showed that compared with GEM alone, both the GEM plus CAP group (OR =1.61; 95% CI: 1.22 to 2.13; P=0.0009) and the GEM plus S-1 group (OR =2.65; 95% CI: 1.99 to 3.52; P<0.00001) could significantly improve ORR, but the GEM plus CIS group (OR =1.40; 95% CI: 0.93 to 2.11; P=0.11) did not achieve same effect.
One-year survival rate
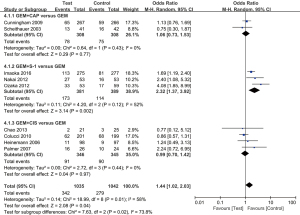
A total of 9 studies containing 2,077 patients reported the 1-year survival rate. We used the random effects model due to the significant heterogeneity (I2=58%; P=0.01). Although the overall meta-analysis showed that GEM-based combination therapy could significantly increase 1-year survival rate (OR =1.44; 95% CI: 1.02 to 2.03; P=0.04) (Figure 7), the subgroup analysis showed that compared with GEM alone, only the GEM plus S-1 Group (OR =2.32; 95% CI: 1.37 to 3.92; P=0.002) could significantly increase 1-year survival rate, but the GEM plus CAP Group (OR =1.06; 95% CI: 0.73 to 1.53; P=0.77) and GEM plus CIS group (OR =0.99; 95% CI: 0.70 to 1.42; P=0.97) did not.
AEs
The pooled AEs (grade ≥3) were summarized in Table 2. The AEs mainly included hematological toxicity and non-hematological toxicity. Common hematological toxicity included leukopenia, neutropenia, thrombocytopenia, and anemia; non-hematological toxicity included nausea, vomiting, diarrhea, constipation, anorexia, and stomatitis. As compared with GEM alone, GEM-based combination therapy had a significantly higher incidence of leukopenia (OR =2.25; 95% CI: 1.67 to 3.01; P<0.00001), neutropenia (OR =1.93; 95% CI: 1.61 to 2.32; P<0.00001), anemia (OR =1.40; 95% CI: 1.05 to 1.86; P=0.02), nausea/vomiting (OR =1.97; 95% CI: 1.43 to 2.72; P<0.0001), diarrhea/constipation (OR =1.68; 95% CI: 1.08 to 2.62; P=0.02), and stomatitis (OR =4.44; 95% CI: 2.00 to 9.87; P=0.003), yet there was no difference in the incidence of thrombocytopenia (OR =1.26; 95% CI: 0.96 to 1.64; P=0.09) and anorexia (OR =1.17; 95% CI: 0.73 to 1.89; P=0.51).
Table 2
| AEs | Subgroup | Studies | Subgroup OR (95% CI) | Subgroup P value | Pooled OR (95% CI) | Pooled P value |
|---|---|---|---|---|---|---|
| Hematological | ||||||
| Leukopenia | GEM + CAP vs. GEM | 1 | 1.33 (0.28, 6.39) | 0.720 | 2.25 (1.67, 3.01) | <0.00001 |
| GEM + S-1 vs. GEM | 3 | 2.62 (1.85, 3.71) | <0.00001 | |||
| GEM + CIS vs. GEM | 4 | 1.56 (0.87, 2.81) | 0.130 | |||
| Neutropenia | GEM + CAP vs. GEM | 4 | 1.61 (1.20, 2.16) | 0.001 | 1.93 (1.61, 2.32) | <0.00001 |
| GEM + S-1 vs. GEM | 4 | 2.34 (1.77, 3.09) | <0.00001 | |||
| GEM + CIS vs. GEM | 4 | 1.83 (1.19, 2.80) | 0.006 | |||
| Thrombocytopenia | GEM + CAP vs. GEM | 4 | 0.45 (0.27, 0.76) | 0.003 | 1.26 (0.96, 1.64) | 0.090 |
| GEM + S-1 vs. GEM | 4 | 2.03 (1.33, 3.11) | 0.001 | |||
| GEM + CIS vs. GEM | 4 | 1.86 (1.09, 3.20) | 0.020 | |||
| Anemia | GEM + CAP vs. GEM | 4 | 1.43 (0.83, 2.45) | 0.200 | 1.40 (1.05, 1.86) | 0.020 |
| GEM + S-1 vs. GEM | 4 | 1.26 (0.85, 1.87) | 0.240 | |||
| GEM + CIS vs. GEM | 4 | 1.78 (0.93, 3.40) | 0.080 | |||
| Non-hematological | ||||||
| Nausea/vomiting | GEM + CAP vs. GEM | 4 | 1.24 (0.79, 1.93) | 0.350 | 1.97 (1.43, 2.72) | <0.0001 |
| GEM + S-1 vs. GEM | 4 | 3.12 (1.57, 6.20) | 0.001 | |||
| GEM + CIS vs. GEM | 4 | 3.44 (1.70, 6.95) | 0.0006 | |||
| Diarrhea/constipation | GEM + CAP vs. GEM | 4 | 1.67 (0.87, 3.21) | 0.120 | 1.68 (1.08, 2.62) | 0.020 |
| GEM + S-1 vs. GEM | 4 | 2.95 (1.20, 7.28) | 0.020 | |||
| GEM + CIS vs. GEM | 4 | 0.92 (0.38, 2.23) | 0.850 | |||
| Anorexia | GEM + CAP vs. GEM | NR | – | – | 1.17 (0.73, 1.89) | 0.510 |
| GEM + S-1 vs. GEM | 4 | 1.08 (0.66, 1.77) | <0.001 | |||
| GEM + CIS vs. GEM | 1 | 4.02 (0.45, 36.29) | 0.220 | |||
| Stomatitis | GEM + CAP vs. GEM | 3 | 4.02 (1.13, 14.33) | 0.030 | 4.44 (2.00, 9.87) | 0.0003 |
| GEM + S-1 vs. GEM | 4 | 7.41 (1.67, 32.86) | 0.008 | |||
| GEM + CIS vs. GEM | 2 | 2.63 (0.60, 11.50) | 0.200 | |||
AEs, adverse events; GEM, gemcitabine; CAP, capecitabine; CIS, cisplatin; NR, not reported; OR, odds ratio; CI, confidence interval.
Publication bias
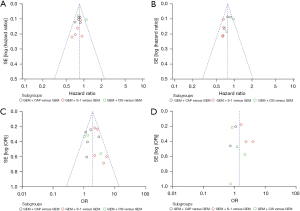
Funnel plot analysis and Egger’ test for the outcomes of OS, PFS, ORR, and 1-year survival rate were performed to explore the publication bias. The plots showed no obvious asymmetry, and the P value of Egger’ test for all outcomes were more than 0.10, suggesting that no publication bias existed (Figure 8).
Discussion
Pancreatic cancer is a common malignant tumor in the digestive system. Due to its occult onset, rapid development, and high degree of malignancy, although the diagnosis of pancreatic cancer has been improved compared with the previous diagnosis, it remains difficult to diagnose and it is still inevitable that patients will be diagnosed in the advanced stage (37,38). Most pancreatic cancer patients are in advanced stage or have distant metastasis at the time of seeing a doctor and have lost the opportunity for surgery. The treatment of these patients can only be palliative, based on chemotherapy to improve the quality of life and survival time of patients. The cytosine nucleoside derivative, GEM, is an antimetabolic and antitumor drug. It is a water-soluble analog of deoxycytidine, which can inhibit cell replication and ribonucleotide reductase, thus inhibiting DNA synthesis and repair (39). It has a good therapeutic effect on pancreatic cancer. Since the clinical trial in 1992, GEM has been the basic drug for chemotherapeutic treatment of pancreatic cancer, especially for patients who cannot undergo surgery, with certain benefits (40). However, due to the limited clinical benefits of GEM and substantial toxic and side effects, including bone marrow suppression, toxic and side effects of digestive system, hepatorenal toxicity, and allergic reaction, it is very challenging for both patients and clinicians. Therefore, GEM-based treatment combined with other drugs is proposed for use in the clinic (41,42).
This study conducted multiple searches within multiple medical databases, and screened them in strict accordance with the pre-established inclusion criteria. The evaluation of literature quality requires that there is no great risk of bias, so the original studies included in the analysis were high-quality clinical studies. The funnel plots were basically symmetrical and evenly dispersed, suggesting that the possibility of publication bias was small. Therefore, this study had high reliability. A meta-analysis of 5,197 patients from 17 RCTs showed that GEM-based combination therapy improved OS and PFS, which had obvious survival advantages, and the ORR and 1-year survival rate were also higher than those of GEM alone. It showed obvious survival benefits, which supported that GEM-based combination therapy can effectively alleviate the disease progression and prolong the life of patients. However, subgroup analysis showed that GEM plus CAP and GEM plus S-1 could effectively improve OS, PFS, and ORR, but GEM plus CIS did not achieve the same effect. Li et al.’s meta-analysis compared the effects of GEM plus fluorouracil-based drugs (CAP and S-1) with GEM alone in advanced pancreatic cancer, and they concluded that compared with GEM alone, GEM combined with fluorouracil significantly improved OS and increased 1-year survival and ORR in patients with advanced pancreatic cancer (43). Zhou et al.’s meta-analysis compared the effects of GEM plus CIS and GEM alone in advanced pancreatic cancer. Their results showed that GEM plus CIS could benefit patients in ORR, but could not make patients obtain better clinical efficacy and long-term prognosis than GEM alone (44). Our study was consistent with the conclusions of the above two studies.
In terms of drug toxicity and side effects, our study showed that GEM-based combination therapy increased various toxic and side effects, mainly in the hematological system and digestive system. However, its incidence was not high, and these toxic and side effects can be predicted and controlled (45,46). Platinum- and fluorouracil-based drugs have great cytotoxicity, so they were found unsuitable for elderly patients with late pancreatic cancer treatment and poor physical fitness. Therefore, GEM plus CIS only had potential benefits in patients with better physical fitness. Studies have shown that fluorouracil drugs had better biological selectivity, tolerance, and lower toxicity and side effects than platinum drugs, and can be used in patients who are not suitable for combination therapy with platinum or other drugs (47,48).
It has been found that GEM also has toxic and side effects of bone marrow suppression. Due to the limited efficacy of GEM alone and the emergence of toxic and side effects and drug resistance of high-dose use, many researchers continue to explore the combination of drugs in the treatment of cancer (49). Most researchers believe that two or more synergistic anticancer drugs can reduce the toxicity and side effects of single drug use and reduce the generation of single drug resistance to a certain extent, improve the metabolic dynamics of drugs in vivo, improve the therapeutic effect of drugs, and reduce the side effects of drugs. Shi et al. retrospectively evaluated the clinical effect of arterial infusion of GEM hydrochloride and fluorouracil drugs for advanced pancreatic cancer, and found that it can obtain better clinical benefits and improve the survival time of patients (50). Shu et al. compared the toxicity and side effects and short-term efficacy of GEM hydrochloride plus fluorouracil and GEM hydrochloride plus CIS for advanced pancreatic cancer. The results showed that the former had a higher clinical benefit response rate and no significant difference in side effects (51).
In clinical practice research, we continuously optimize the treatment plan, from the initial single drug treatment to the combined treatment plan, and the new adjuvant treatment before cancer surgery, continuously promote the treatment process of pancreatic cancer, and improve the quality of life of patients with pancreatic cancer (52). With the development of tumor molecular biology, more and more therapeutic targets will be discovered and recognized. Improving the effective rate without increasing toxicity is the development direction of targeted therapy. It is believed that in the future, GEM synthetic preparations with low toxicity, good therapeutic effect, and long half-life will be developed to play a better role in the first line of anti-cancer.
This study had several shortcomings. Firstly, some of the selected studies were non-blind trials, and the research quality was slightly deficient. Secondly, most of the studies included only Asian patients were (mostly in China and Japan) in the GEM plus S-1 group, and there was no research comparison on ethnic differences. Therefore, the results may only be applicable to Asian patients; whether the research results are applicable to patients in other regions needs more research to confirm. Finally, the included literature did not provide detailed data on the quality-of-life scale and cost-effectiveness, so the project could not be analyzed. It can be speculated that the treatment cost of the combined chemotherapy group will certainly increase due to the increased use of chemotherapeutic drugs and the subsequent occurrence of more side effects.
Conclusions
This meta-analysis showed that OS, PFS, 1-year survival rate, and ORR of GEM-based combination therapy were statistically significantly improved, although the AEs were also increased. Subgroup analysis showed that the efficacy of GEM plus CAP and GEM plus S-1 was better than that of GEM alone, while GEM plus CIS did not show superiority. The existing evidence suggested that the combination therapy had better efficacy and higher survival benefit than GEM alone. However, considering the high rate of AEs and latent economic problems, clinicians need to consider the patient’s condition, treatment willingness, and financial situation. In addition, how to further reduce AEs is worthy of further study.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-624/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-624/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirai I, Kimura W, Ozawa K, et al. Perineural invasion in pancreatic cancer. Pancreas 2002;24:15-25. [Crossref] [PubMed]
- Sarvepalli D, Rashid MU, Rahman AU, et al. Gemcitabine: a review of chemoresistance in pancreatic cancer. Crit Rev Oncog 2019;24:199-212. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA 2021;326:851-62. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Tsang ES, Spratlin J, Cheung WY, et al. Real-world Outcomes Among Patients Treated With Gemcitabine-based Therapy Post-FOLFIRINOX Failure in Advanced Pancreatic Cancer. Am J Clin Oncol 2019;42:903-8. [Crossref] [PubMed]
- Zhang XW, Ma YX, Sun Y, et al. Gemcitabine in Combination with a Second Cytotoxic Agent in the First-Line Treatment of Locally Advanced or Metastatic Pancreatic Cancer: a Systematic Review and Meta-Analysis. Target Oncol 2017;12:309-21. [Crossref] [PubMed]
- Ishimoto U, Kinoshita A, Hirose Y, et al. The efficacy and safety of nab paclitaxel plus gemcitabine in elderly patients over 75 years with unresectable pancreatic cancer compared with younger patients. Cancer Chemother Pharmacol 2019;84:647-54. [Crossref] [PubMed]
- Inal A, Kos FT, Algin E, et al. Gemcitabine alone versus combination of gemcitabine and cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a retrospective analysis of multicenter study. Neoplasma 2012;59:297-301. [Crossref] [PubMed]
- Kasi A, McGinnis T, Naik G, et al. Efficacy and tolerability of the combination of nano-liposomal irinotecan and 5-fluorouracil/leucovorin in advanced pancreatic adenocarcinoma: post-approval clinic experience. J Gastrointest Oncol 2021;12:464-73. [Crossref] [PubMed]
- Li Q, Yan H, Liu W, et al. Efficacy and safety of gemcitabine-fluorouracil combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomized controlled trials. PLoS One 2014;9:e104346. [Crossref] [PubMed]
- Ouyang G, Wu Y, Liu Z, et al. Efficacy and safety of gemcitabine-capecitabine combination therapy for pancreatic cancer: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2021;100:e27870. [Crossref] [PubMed]
- Fumet JD, Vincent J, Bengrine L, et al. Safety and Efficacy of Gemcitabine, Docetaxel, Capecitabine, Cisplatin as Second-line Therapy for Advanced Pancreatic Cancer After FOLFIRINOX. Anticancer Res 2020;40:4011-5. [Crossref] [PubMed]
- Hagiwara Y, Ohashi Y, Okusaka T, et al. Health-related quality of life in a randomised phase III study of gemcitabine plus S-1, S-1 alone and gemcitabine alone for locally advanced or metastatic pancreatic cancer: GEST study. ESMO Open 2017;2:e000151. [Crossref] [PubMed]
- Ottaiano A, Capozzi M, De Divitiis C, et al. Gemcitabine mono-therapy versus gemcitabine plus targeted therapy in advanced pancreatic cancer: a meta-analysis of randomized phase III trials. Acta Oncol 2017;56:377-83. [Crossref] [PubMed]
- Waissi W, Amé JC, Mura C, et al. Gemcitabine-Based Chemoradiotherapy Enhanced by a PARP Inhibitor in Pancreatic Cancer Cell Lines. Int J Mol Sci 2021;22:6825. [Crossref] [PubMed]
- Cascinu S, Berardi R, Bianco R, et al. Nab-paclitaxel/gemcitabine combination is more effective than gemcitabine alone in locally advanced, unresectable pancreatic cancer - A GISCAD phase II randomized trial. Eur J Cancer 2021;148:422-9. [Crossref] [PubMed]
- Fukahori M, Miwa K, Murotani K, et al. A phase II study of gemcitabine plus nab-paclitaxel as first-line therapy for locally advanced pancreatic cancer. Medicine (Baltimore) 2021;100:e26052. [Crossref] [PubMed]
- Riedl JM, Posch F, Horvath L, et al. Gemcitabine/nab-Paclitaxel versus FOLFIRINOX for palliative first-line treatment of advanced pancreatic cancer: A propensity score analysis. Eur J Cancer 2021;151:3-13. [Crossref] [PubMed]
- Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 2010;28:1645-51. [Crossref] [PubMed]
- Scheithauer W, Schüll B, Ulrich-Pur H, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol 2003;14:97-104. [Crossref] [PubMed]
- Chao Y, Wu CY, Wang JP, et al. A randomized controlled trial of gemcitabine plus cisplatin versus gemcitabine alone in the treatment of metastatic pancreatic cancer. Cancer Chemother Pharmacol 2013;72:637-42. [Crossref] [PubMed]
- de Jong EJM, Janssen QP, Simons TFA, et al. Real-world evidence of adjuvant gemcitabine plus capecitabine vs gemcitabine monotherapy for pancreatic ductal adenocarcinoma. Int J Cancer 2022;150:1654-63. [Crossref] [PubMed]
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24:3946-52. [Crossref] [PubMed]
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. [Crossref] [PubMed]
- Ozaka M, Matsumura Y, Ishii H, et al. Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol 2012;69:1197-204. [Crossref] [PubMed]
- Sudo K, Ishihara T, Hirata N, et al. Randomized controlled study of gemcitabine plus S-1 combination chemotherapy versus gemcitabine for unresectable pancreatic cancer. Cancer Chemother Pharmacol 2014;73:389-96. [Crossref] [PubMed]
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-8. [Crossref] [PubMed]
- Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007;25:2212-7. [Crossref] [PubMed]
- Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer 2002;94:902-10. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Imaoka H, Kou T, Tanaka M, et al. Clinical outcome of elderly patients with unresectable pancreatic cancer treated with gemcitabine plus S-1, S-1 alone, or gemcitabine alone: Subgroup analysis of a randomised phase III trial, GEST study. Eur J Cancer 2016;54:96-103. [Crossref] [PubMed]
- Bernhard J, Dietrich D, Scheithauer W, et al. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial--SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol 2008;26:3695-701. [Crossref] [PubMed]
- Lee HS, Chung MJ, Park JY, et al. A randomized, multicenter, phase III study of gemcitabine combined with capecitabine versus gemcitabine alone as first-line chemotherapy for advanced pancreatic cancer in South Korea. Medicine (Baltimore) 2017;96:e5702. [Crossref] [PubMed]
- Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol 2007;14:2088-96. [Crossref] [PubMed]
- Nakai Y, Isayama H, Sasaki T, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer 2012;106:1934-9. [Crossref] [PubMed]
- Katz MHG. Borderline resectable pancreatic cancer: Need for standardization and quality control in clinical treatment trials. Pancreatology 2016;4:S9. [Crossref]
- Haddad PA, Gallagher KM, Hammoud D. Comparative efficacy of commonly used first-line chemotherapy regimens in advanced pancreatic cancer: A network meta-analysis. J Clin Oncol 2021;39:abstr 403.
- Zhou X, Huang Z, Xu L, et al. A panel of 13-miRNA signature as a potential biomarker for predicting survival in pancreatic cancer. Oncotarget 2016;7:69616-24. [Crossref] [PubMed]
- Richards KE, Zeleniak AE, Fishel ML, et al. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017;36:1770-8. [Crossref] [PubMed]
- Ouyang G, Liu Z, Huang S, et al. Gemcitabine plus cisplatin versus gemcitabine alone in the treatment of pancreatic cancer: a meta-analysis. World J Surg Oncol 2016;14:59. [Crossref] [PubMed]
- Zhang D, Zheng Y, Guo X, et al. Meta-analysis of the clinical efficacy of FOLFIRINOX in the treatment of inoperable advanced pancreatic cancer and gemcitabine-based regimen. J Adv Health 2019;1:281-6. [Crossref]
- Li D, Chen C, Zhou Y, et al. Gemcitabine Compared With Gemcitabine and S-1 Combination Therapy in Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e1345. [Crossref] [PubMed]
- Zhou TY, He XH, Tan ZH, et al. The gemcitabine plus cisplatin is not beneficial for patients with advanced pancreatic cancer: meta analysis. Journal of Modern Oncology 2019;27:2546-52.
- Hamada C, Okusaka T, Ikari T, et al. Efficacy and safety of gemcitabine plus S-1 in pancreatic cancer: a pooled analysis of individual patient data. Br J Cancer 2017;116:1544-50. [Crossref] [PubMed]
- Papneja N, Ahmed S, Mondal P, et al. Outcomes of first-line FOLFIRINOX (FFX) versus gemcitabine and nab-paclitaxel (GN) in patients with advanced pancreatic cancer: Multi-Institutional Canadian sites experience. Ann Oncol 2019;30:v267. [Crossref]
- Bramhall SR, Allum WH, Jones AG, et al. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg 1995;82:111-5. [Crossref] [PubMed]
- Xiao BY, Wang BC, Lin GH, et al. Efficacy and safety of gemcitabine plus capecitabine in the treatment of advanced or metastatic pancreatic cancer: a systematic review and meta-analysis. Ann Palliat Med 2020;9:1631-42. [Crossref] [PubMed]
- Blomstrand H, Green H, Fredrikson M, et al. Clinical characteristics and blood/serum bound prognostic biomarkers in advanced pancreatic cancer treated with gemcitabine and nab-paclitaxel. BMC Cancer 2020;20:950. [Crossref] [PubMed]
- Shi XY, Zhao J. Research progress of Gemcitabine in combined therapy of pancreatic cancer. China Medical Herald 2020;17:50-3.
- Shu YC, Li YQ, Zhang QX. Comparison of Clinical Efficacy and Toxicity Between Gemcitabine Plus S-1 and Gemcitabine in the Treatment of Advanced Pancreatic Cancer. Clinical Medical & Engineering 2015;22:1471-2.
- Zhou J, Zhao R, Wen F, et al. Cost-effectiveness analysis of gemcitabine, S-1 and gemcitabine plus S-1 for treatment of advanced pancreatic cancer based on GEST study. Med Oncol 2015;32:121. [Crossref] [PubMed]
(English Language Editor: J. Jones)





