
Anti-PD-1 antibodies plus lenvatinib in patients with unresectable hepatocellular carcinoma who progressed on lenvatinib: a retrospective cohort study of real-world patients
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the fourth leading cause of cancer-related death worldwide (1). Most patients are diagnosed with advanced or unresectable HCC; hence, systemic treatment is the major choice for these patients (2,3). Furthermore, the disease recurs in about 70% of patients who received surgical resection of the primary tumor (4).
With the progression of new tyrosine kinase inhibitors (TKIs) and immune therapy, the therapeutic options for advanced unresectable HCC have progressed dramatically in recent years (2). For patients with HCC, lenvatinib, an oral multi-targeted TKI, is non-inferior to sorafenib in terms of overall survival (OS) and objective response rate (ORR), and significantly improves the progression-free survival (PFS) of patients (5). However, the effectiveness of lenvatinib is limited by drug resistance and intolerable toxicities (6). Recently, several molecular targeted agents have been approved for the treatment of HCC, such as regorafenib, cabozantinib, and ramucirumab (for patients with serum alpha-fetoprotein ≥400 ng/mL) (7). However, there is no evidence of significant effect of these drugs for patients who have progressed with lenvatinib (8).
HCC is highly heterogeneous and multiple molecular pathways are involved in the development of drug resistance in HCC cells (9). Combining TKIs and immune checkpoint inhibitors (ICIs) is one way to potentially boost effectiveness and minimize treatment resistance in HCC (10). Recently, the safety and efficacy of the combination of TKIs and anti-programmed cell death protein-1 (PD-1) antibodies have been reported in several studies (11). Treatment with lenvatinib plus anti-PD-1 immunotherapy was associated with good survival outcomes in patients with unresectable HCC (12). Surprisingly, in an open-label, multicenter study, treatment with lenvatinib plus pembrolizumab was associated with a median OS of 22 months in patients with unresectable HCC (13). Treatment after the progression of the TKI therapy is important, which is closely related to the overall survival. Although second-line treatment options following progression on sorafenib have been established (14), effective treatments following progression on lenvatinib have not been fully elucidated. There is no standard follow-up treatment regimen for patients with advanced HCC who have failed lenvatinib therapy. Because of the comprehensive coverage of therapeutic targets of lenvatinib, the remission rate of other TKI treatments in HCC patients resistant to lenvatinib is quite low. At present, it is unknown whether the addition of immunotherapy whilst continuing lenvatinib could benefit patients.
We present the results of this retrospective, real-world study to evaluate the effectiveness and tolerability of the combination of anti-PD-1 antibodies and lenvatinib in Chinese patients with unresectable HCC who had progressed on lenvatinib. We present the following article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-643/rc).
Methods
Patients
Patients were eligible for inclusion in this real-world study if they had HCC that was initially considered unresectable [according to the American Association for the Study of Liver Diseases (AASLD) guidelines (15)], had received oral lenvatinib (≥4 mg/day for at least 1 month) and experienced disease progression during lenvatinib treatment, and then received anti-PD-1 antibodies (pembrolizumab or toripalimab) combined with lenvatinib between April 2018 and April 2020 at the Zhongshan Hospital, Fudan University, Shanghai, China. Patients were also required to have a tumor classification of Barcelona Clinic Liver Cancer (BCLC) B or C, a Child-Pugh score ≤7, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, more than one measurable target lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 (16), and complete medical and follow-up data available.
Patients who received other systemic anti-cancer therapies concomitantly with anti-PD-1 antibodies plus lenvatinib and those with malignant tumors other than HCC, immunodeficiency, or active autoimmune disease, were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University (No. B2019-296R). Individual consent for this retrospective analysis was waived.
Study design
As is standard practice at our institution, lenvatinib was given orally at a dose of 12 mg/day for bodyweight ≥60 kg and 8 mg/day for bodyweight <60 kg. Standard doses of anti-PD-1 antibodies (pembrolizumab 200 mg or toripalimab 240 mg) were administered intravenously every 3 weeks to patients who experienced disease progression while receiving oral lenvatinib.
Clinical and laboratory data were retrospectively collected from the charts of eligible patients. The patients’ demographics, tumor responses, and health-related quality of life (HRQOL) data were also collected.
According to standard practice, patients were followed-up every 6–8 weeks using enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and laboratory tests, and anti-tumor effects were assessed according to RECIST v1.1 (16). To evaluate the safety of anti-PD-1 antibodies plus lenvatinib, treatment-related adverse reactions (TRAEs) were investigated according to the patient’s treatment time and the laboratory test time. PFS and OS were calculated from the date of treatment initiation with an anti-PD-1 antibody plus lenvatinib to the time of tumor progression or the patient’s death, respectively.
Two questionnaires [Quality of Life Questionnaire Core 30 (QLQ C-30) and Quality of Life Questionnaire Hepatocellular Carcinoma 18 (QLQ HCC18)] from the European Organization for Research and Treatment of Cancer (EORTC) were used to assess the HRQOL (17-19), according to the standard practice at our institution. These questionnaires were conducted immediately before and after anti-PD-1 antibodies plus lenvatinib treatment.
The EORTC QLQ-C30 contains 30 questions grouped into five functional domains (physical, role, emotional, cognitive, and social), three symptom domains (fatigue, pain, nausea and vomiting), six individual symptom items, and one scale assessing the global quality of life. The QLQ-HCC18 contains 18 questions grouped into eight domains (fatigue, jaundice, nutrition, pain, fever, body image, abdominal swelling, and sex life) (18). All questionnaire responses were transformed into scores ranging from 0 to 100. The total score was only calculated when the scores for all domains/items were available.
Statistical analysis
Continuous variables were described by the mean ± standard error (SE) and between-group differences were compared using the Student’s t-test. Categorical variables were described as frequencies and percentages. The chi-squared test was used to evaluate the response to treatment, and paired Student’s t-test was used to compare laboratory parameters and HRQOL before and after lenvatinib treatment. Survival was analyzed using the Kaplan-Meier method, and differences in the survival curves were estimated using a log-rank test. P<0.05 was considered statistically significant. All data analyses were performed using SPSS software (v25.0; SPSS Inc., Chicago, IL, USA).
Results
Patients
A total of 46 patients were enrolled in this study, including 41 (89.1%) males and 5 (10.8%) females, with a median age of 58 (range 45–69) years (Table 1). The main characteristics of the study population are shown in Table 1. The most common etiology of liver disease among the patients was the hepatitis B virus (HBV & both B and C; n=44, 95.7%). All patients were classified as Child-Pugh class A. Overall, 12 (26.1%) patients were in BCLC B stage and 34 (73.9%) patients were in BCLC C stage. The ECOG performance status score was 0 in 30 patients (65.2%) and 1 in 16 patients (34.8%). Macrovascular invasion was present in 15 (32.6%) patients. Of the 26 (56.5%) patients with distant metastasis, seven had lung metastases, five had bone metastases, and 13 had metastases at other sites. Most patients (n=31; 67.4%) had only previously received lenvatinib, while 15 (32.6%) patients had previously received sorafenib or chemotherapy, and had received lenvatinib as a second- or third-line systemic treatment.
Table 1
| Variables | Patients, n (%) |
|---|---|
| Age (years), median [range] | 58 [45–69] |
| Sex | |
| Male | 41 (89.0) |
| Female | 5 (11.0) |
| Weight (kg), median [range] | 65 [49–82] |
| ECOG performance status | |
| 0 | 30 (65.2) |
| 1 | 16 (34.8) |
| Child-Pugh score | |
| 5 | 24 (52.2) |
| 6 | 22 (47.8) |
| 7 | 0 |
| BCLC stage | |
| B | 12 (26.1) |
| C | 34 (73.9) |
| Etiology of chronic liver disease | |
| Hepatitis B | 43 (93.4) |
| Hepatitis C | 1 (2.2) |
| Both hepatitis B and C | 1 (2.2) |
| Other | 1 (2.2) |
| MVI (PVVT) | 15 (32.6) |
| vp1 | 5 (10.9) |
| vp2 | 1 (2.2) |
| vp3 | 4 (8.7) |
| vp4 | 5 (10.9) |
| Number of tumors | |
| ≤3 | 18 (39.1) |
| >3 | 28 (60.9) |
| Maximum diameter of tumor (cm) (mean ± SD) | 4.7±4.2 |
| Extra-hepatic spread | 26 (56.5) |
| Lung | 7 (15.2) |
| Bone | 5 (10.9) |
| Mesentery | 3 (6.5) |
| Other | 11 (23.9) |
| Previous systemic therapy | |
| Lenvatinib | 31 (67.4) |
| Sorafenib | 15 (32.6) |
BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; MVI, microvascular invasion; PVVT, portal vein tumor thrombus.
Twenty-nine patients received lenvatinib 8 mg/day and seven patients received 12 mg/day; the remaining 20 patients received lenvatinib 4–12 mg/day during the study due to adverse events. The median duration of lenvatinib treatment before the study was 4.1 [interquartile range (IQR), 1.8–8.1] months, while that of combination treatment was 5.7 (IQR, 2.0–12.0) months.
Tumor response and survival
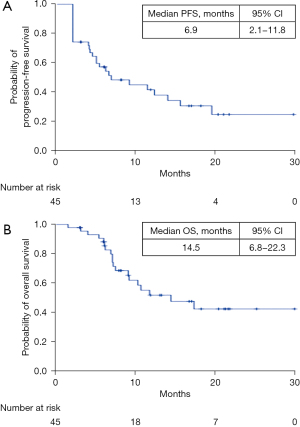
The ORR and the disease control rate (DCR) were 23.9% (11/46) and 71.7% (33/46), respectively (RECIST 1.1). After a median follow-up period of 15.6 (IQR, 11.2–22.0) months, the median PFS and median OS were 6.9 [95% confidence interval (CI): 2.1–11.8] months and 14.5 (95% CI: 6.8–22.3) months, respectively (Figure 1).
Safety
The most common TRAEs were decreased thyroid function (43.5%), loss of appetite (43.5%), hypertension (36.9%), fatigue (34.8%), and diarrhea (26.1%) (Table 2). Grade 3/4 adverse events occurred in 16 patients (34.8%), which included gastrointestinal hemorrhage, anorexia, and hypertension (Table 2).
Table 2
| TRAEs | Any grade, n (%) | Grade ≥3, n (%) |
|---|---|---|
| Appetite loss | 20 (43.5) | 4 (8.7) |
| Hypothyroidism | 20 (43.5) | 1 (2.2) |
| Hypertension | 17 (36.9) | 3 (6.5) |
| Fatigue | 16 (34.8) | 0 |
| Diarrhea | 12 (26.1) | 2 (4.3) |
| Dysphonia | 10 (21.7) | 0 |
| Weight loss | 9 (19.6) | 0 |
| Hand-foot syndrome | 8 (17.4) | 0 |
| Proteinuria | 8 (17.4) | 1 (2.2) |
| Hemorrhage | – | 5 (10.9) |
| Immune pneumonia | – | 2 (4.3) |
| Immune enteritis | – | 1 (2.2) |
| Immune hepatitis | – | 1 (2.2) |
| Rash | – | 1 (2.2) |
| Shoulder pain | – | 1 (2.2) |
| Thrombocytopenia | – | 1 (2.2) |
Table contains any TRAE reported in >5 (10%) patients and any TRAE ≥ grade 3. TRAEs, treatment-related adverse events.
Dose reductions were required in 19 (41.3%) patients, and one (2%) patient discontinued treatment due to diabetic ketoacidosis. Immune-related adverse events occurred in 17 (36.9%) patients, and included interstitial pneumonia (n=2), upper gastrointestinal hemorrhage (n=2), immune enteritis (n=1), grade 4 thrombocytopenia (n=1), diabetes (n=1), and 10 additional grade 1/2 adverse events.
HRQOL
Both the EORTC QLQ-C30 and QLQ-HCC18 questionnaires were completed by 58.7% (27/46) of patients. Emotional functioning and overall HRQOL were improved significantly in patients with HCC after initiation of treatment with anti-PD-1 antibodies plus lenvatinib (Table 3). Compared to lenvatinib monotherapy, anti-PD-1 antibodies plus lenvatinib reduced the incidence of deterioration on the EORTC QLQ-HCC18 disease-specific symptom scales. Compared with lenvatinib alone, the addition of anti-PD-1 antibodies to lenvatinib significantly reduced fatigue [4.9±7.5 vs. 11.1±12.7 (QLQL-HCC18), hazard ratio (HR) 0.60, 95% CI: 0.45–0.80], diarrhea [12.3±20.9 vs. 18.5±16.8 (QLQ-C30), HR 0.65, 95% CI: 0.46–0.92], and pain [5.5±10.3 vs. 11.1±13.9 (QLQL-HCC18), HR 0.62, 95% CI: 0.08–10.04] in patients with unresectable HCC.
Table 3
| Symptoms/problems | Before lenvatinib (n=27) | After lenvatinib (n=27) | After anti-PD-1 antibody (n=27) | P value |
|---|---|---|---|---|
| QLQ-C30 | ||||
| Physical function | 86.7±11.5 | 86.2±4.8 | 84.9±14.7 | 0.41 |
| Role function | 72.2±16.7 | 71.6±22.1 | 74.7±21.9 | 0.30 |
| Emotional function | 91.0±7.6 | 94.1±7.6 | 95.4±6.7 | 0.01* |
| Cognitive function | 93.8±9.4 | 94.4±9.2 | 95.1±7.8 | 0.23 |
| Social function | 75.3±18.7 | 74.1±19.8 | 76.5±20.8 | 0.45 |
| Global HRQOL | 54.1±9.3 | 61.8±11.2 | 67.6±11.6 | 0.00* |
| Fatigue | 16.0±15.0 | 13.9±14.3 | 13.6±13.5 | 0.67 |
| Nausea/vomiting | 4.3±8.8 | 4.3±8.8 | 1.2±4.4 | 0.06 |
| Pain | 9.3±14.1 | 13.0±14.1 | 9.9±15.5 | 0.34 |
| Dyspnea | 3.7±10.7 | 3.7±10.6 | 3.7±14.1 | 1.00 |
| Sleep disturbance | 7.4±14.1 | 4.9±12.1 | 2.5±8.9 | 0.13 |
| Appetite loss | 14.8±16.9 | 19.8±19.1 | 13.6±19.1 | 0.17 |
| Constipation | 2.5±8.9 | 3.7±10.7 | 2.5±8.9 | 0.57 |
| Diarrhea | 4.9±15.2 | 18.5±16.8 | 12.3±20.9 | 0.01* |
| Financial difficulties | 13.6±16.7 | 12.3±18.8 | 13.6±19.1 | 0.75 |
| QLQ-HCC18 | ||||
| Abdominal swelling | 13.6±23.1 | 14.8±23.3 | 14.8±19.2 | 0.95 |
| Body image | 0.6±3.2 | 0.6±3.1 | 0.6±3.2 | 1.00 |
| Jaundice | 0.0±0.0 | 0.6±3.1 | 1.8±5.3 | 0.11 |
| Pain | 4.9±10.1 | 11.1±13.9 | 5.5±10.3 | 0.01* |
| Fever | 0.62±3.2 | 1.8±5.3 | 3.7± 7.1 | 0.16 |
| Nutrition | 0.7±2.8 | 0.7±2.1 | 1.0±2.4 | 0.75 |
| Fatigue | 10.3±11.5 | 11.1±12.7 | 4.9±7.5 | 0.03* |
| Sex life | 3.7±14.1 | 4.9±15.2 | 0.0±0.0 | 0.14 |
Data are presented as scores ± SD in all patients who completed both questionnaires (n=27). The P value represents the comparison between the combination therapy with lenvatinib (i.e., after anti-PD-1 antibody) and monotherapy (after lenvatinib). Scores range from 0–100. In the QLQ-C30, for functional (physical, role, emotional, cognitive and social) or global HRQOL, higher scores represent a relatively higher/healthier level of functioning or global QoL. For the symptom/problem scale, higher scores represent more severe symptoms/problems. In the QLQ-HCC18, higher scores indicated worse symptoms. *, P<0.05. QLQ-30, Quality of Life Questionnaire Core 30; QLQ-HCC18, Quality of Life Questionnaire Hepatocellular Carcinoma 18; QoL, quality of life; SD, standard deviation.
Discussion
With the emergence of new TKIs and immune therapy, the therapeutic options for advanced HCC have progressed dramatically in recent years. Currently, sorafenib and lenvatinib are first-line therapies for unresectable HCC. While many effective molecular targeted agents have become available, such as regorafenib (20), cabozantinib (21), and ramucirumab (22) (for patients with a serum alpha-fetoprotein (AFP) level ≥400 ng/mL), second-line options have been established after progression on sorafenib. However, effective treatments after progression on lenvatinib have not been fully elucidated.
Lenvatinib and anti-PD-1 inhibitors have proven survival benefits when used as a first- or second-line therapy for advanced HCC. Recently, lenvatinib plus pembrolizumab has been shown to be a potent systemic combination therapy in patients with unresectable HCC (23). However, there were no data regarding the benefits of combined anti-PD-1 antibodies with lenvatinib in patients who had previously progressed on lenvatinib. Therefore, we assessed the effectiveness and safety of immune checkpoint inhibitors plus lenvatinib in real-world patients with unresectable HCC who had progressed on lenvatinib.
In this real-world study of combined ICIs with lenvatinib in patients with unresectable HCC who had progressed on lenvatinib, the ORR and DCR were 23.9% (11/46) and 71.7% (33/46), respectively, based on RECIST 1.1. After a median follow-up period of 15.6 (IQR, 11.2–22.0) months, the median PFS and OS were 6.9 (95% CI: 2.1–11.8) months and 14.5 (95% CI: 6.8–22.3) months, respectively. In the Keynote-524 trial (13) of lenvatinib plus pembrolizumab in patients with advanced HCC, the ORR and DCR were 36.0% (95% CI: 26.6–46.2%) and 88.0% (95% CI: 80.0–93.6), respectively, according to RECIST 1.1 using independent imaging review. Moreover, in this open-label multicenter study, lenvatinib combined with pembrolizumab showed a median PFS of 8.6 months and a median OS of 22 months (13). However, lenvatinib plus pembrolizumab were used as a first-line treatment in the Keynote-524 trial. Patients in our study had already progressed on lenvatinib. In the REFLECT study, the median PFS for first-line lenvatinib in HCC was 7.4 months (24). In the present study, we found that the median PFS of patients on PD-1 antibodies plus lenvatinib was 6.9 months after progression on lenvatinib monotherapy.
Excluding the performance of lenvatinib in the REFLECT study, the ORR for molecular targeted drug monotherapy is generally less than 25% (25). The survival of patients with unresectable HCC is approximately 1 year (26), and thus, the overall efficacy of molecular targeted drug monotherapy is not ideal. Additionally, ICI monotherapy is not very effective in HCC, with ORRs below 20% (27). In two large phase III clinical trials, CheckMate-459 (28) and KEYNOTE-240 (29), the OS of patients on ICI monotherapy (either first- or second-line) had no statistical significance compared to that of patients in the control groups. More importantly, the proportion of patients with tumor progression after single-agent immunotherapy often exceeds 30% (30). Furthermore, some patients may progress more quickly with immunotherapy—hyperprogression—a notable issue for patients with HCC receiving immunotherapy (31,32). Kim et al. (33) identified a potential protective effect against the development of hyperprogression during PD-1 blockade with anti-vascular endothelial growth factor (VEGF) therapy. Preclinical evidence suggests that combining a TKI inhibitor and anti-PD-1/anti-PD-L1 antibody induces additive anti-tumor effects (34). Lenvatinib, which has anti-VEGF effects, normalizes the tumor vasculature and increases the effective infiltration of T lymphocytes in the immune-therapy microenvironment (35). Previous studies have shown that ICI-mediated antitumor responses depend on the infiltration of T lymphocytes (36). Therefore, lenvatinib can provide an effective microenvironment for anti-PD-1 therapy (23,37).
Clinicians will be understandably concerned that the combination of lenvatinib and ICIs in patients who have failed first-line TKI treatment will increase the side effects. In the current study, combination therapy was relatively well tolerated, with immune-related adverse events experienced by 36.9% of patients and no treatment-related deaths. The most common TRAEs were anorexia (43.5%), hypothyroidism (43.5%), hypertension (36.9%), fatigue (34.8%), and diarrhea (26.1%). Grade 3/4 events occurred in 16 patients (34.8%). These results are similar to those of lenvatinib plus pembrolizumab in the Keynote-524 trial (13), in which the most common TRAEs were anorexia, hypertension, diarrhea, and fatigue, with no unexpected safety signals.
The HRQOL of patients in this study was also evaluated using the QLQ C-30 and QLQ HCC18 questionnaires. HRQOL analysis is important in clinical studies to clarify a clinically meaningful benefit from the patients’ perspective, particularly in cases where there are no cures (38). Emotional functioning and overall HRQOL were significantly improved in patients with HCC following the initiation of anti-PD-1 antibodies plus lenvatinib. The addition of anti-PD-1 antibodies to lenvatinib markedly reduced fatigue, diarrhea, and pain compared with lenvatinib alone in patients with advanced HCC.
The present study has some limitations that should be noted. Firstly, this was a retrospective study with a relatively small sample size, resulting in inevitable selection bias and limited evidence. Moreover, since only patients who had progressed on lenvatinib and subsequently received anti-PD-1 antibodies plus lenvatinib were included, there was no control group (placebo plus lenvatinib following lenvatinib progression). Thus, randomized controlled trials with a larger sample size are needed to demonstrate the benefits of ICIs plus lenvatinib in patients with unresectable HCC who had previously progressed on lenvatinib.
In summary, this study evaluated the effectiveness and safety of anti-PD-1 inhibitors plus lenvatinib in patients with unresectable HCC who progressed on lenvatinib. The combination of PD-1 inhibitors and lenvatinib was well tolerated. Emotional functioning and overall HRQOL in patients with HCC improved significantly following the initiation of anti-PD-1 antibodies plus lenvatinib. The combination of anti-PD-1 antibodies and lenvatinib may benefit patients with advanced HCC who progress on lenvatinib and presents a new potential treatment option.
Acknowledgments
Funding: This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (grant Nos. 81702310 and 81502007).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-643/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-643/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-643/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University (No. B2019-296R). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print. [Crossref] [PubMed]
- Finn RS, Zhu AX. Evolution of Systemic Therapy for Hepatocellular Carcinoma. Hepatology 2021;73:150-7. [Crossref] [PubMed]
- Kudo M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers (Basel) 2018;10:412. [Crossref] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Cavalieri S, Cosmai L, Genderini A, et al. Lenvatinib-induced renal failure: two first-time case reports and review of literature. Expert Opin Drug Metab Toxicol 2018;14:379-85. [Crossref] [PubMed]
- Jindal A, Thadi A, Shailubhai K. Hepatocellular Carcinoma: Etiology and Current and Future Drugs. J Clin Exp Hepatol 2019;9:221-32. [Crossref] [PubMed]
- Marin JJG, Macias RIR, Monte MJ, et al. Molecular Bases of Drug Resistance in Hepatocellular Carcinoma. Cancers (Basel) 2020;12:1663. [Crossref] [PubMed]
- Zhang T, Guan G, Zhang J, et al. E2F1-mediated AUF1 upregulation promotes HCC development and enhances drug resistance via stabilization of AKR1B10. Cancer Sci 2022;113:1154-67. [Crossref] [PubMed]
- Haber PK, Puigvehí M, Castet F, et al. Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002-2020). Gastroenterology 2021;161:879-98. [Crossref] [PubMed]
- Zhu XD, Li KS, Sun HC. Adjuvant therapies after curative treatments for hepatocellular carcinoma: Current status and prospects. Genes Dis 2020;7:359-69. [Crossref] [PubMed]
- Huang C, Zhu XD, Shen YH, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res 2021;9:19. [Crossref] [PubMed]
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020;38:2960-70. [Crossref] [PubMed]
- Villanueva A, Llovet JM. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib. Clin Cancer Res 2012;18:1824-6. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Bonnetain F, Paoletti X, Collette S, et al. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: results from two French clinical trials. Qual Life Res 2008;17:831-43. [Crossref] [PubMed]
- Blazeby JM, Currie E, Zee BC, et al. Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer 2004;40:2439-44. [Crossref] [PubMed]
- Zhao H, Kanda K. Testing psychometric properties of the standard Chinese version of the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire 30 (EORTC QLQ-C30). J Epidemiol 2004;14:193-203. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-96. [Crossref] [PubMed]
- Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:711-8. [Crossref] [PubMed]
- Yamashita T, Kudo M, Ikeda K, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 2020;55:113-22. [Crossref] [PubMed]
- Wang DX, Yang X, Lin JZ, et al. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: A retrospective, real-world study conducted in China. World J Gastroenterol 2020;26:4465-78. [Crossref] [PubMed]
- Seong J, Park HC, Han KH, et al. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys 2003;55:329-36. [Crossref] [PubMed]
- Shrestha R, Bridle KR, Crawford DHG, Jayachandran A. Immune checkpoint blockade therapies for HCC: current status and future implications. Hepatoma Research 2019;5:32. [Crossref]
- Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77-90. [Crossref] [PubMed]
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193-202. [Crossref] [PubMed]
- Sangro B, Sarobe P, Hervás-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525-43. [Crossref] [PubMed]
- Mandal S, Ray B, Baniya Sharma S, et al. Hyperprogression: A Unique Phenomenon of Progression of Existing Tumor Secondary to Immunotherapy. Cureus 2021;13:e17992. [Crossref] [PubMed]
- Chan SL. Hyperprogression in hepatocellular carcinoma: Illusion or reality? J Hepatol 2021;74:269-71. [Crossref] [PubMed]
- Kim CG, Kim C, Yoon SE, et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol 2021;74:350-9. [Crossref] [PubMed]
- Kasikara C, Davra V, Calianese D, et al. Pan-TAM Tyrosine Kinase Inhibitor BMS-777607 Enhances Anti-PD-1 mAb Efficacy in a Murine Model of Triple-Negative Breast Cancer. Cancer Res 2019;79:2669-83. [Crossref] [PubMed]
- Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599-616. [Crossref] [PubMed]
- Naimi A, Mohammed RN, Raji A, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal 2022;20:44. [Crossref] [PubMed]
- Reig M, Bruix J. Lenvatinib: can a non-inferiority trial change clinical practice? Lancet 2018;391:1123-4. [Crossref] [PubMed]
- Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol 2015;16:e32-42. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


