CircMMP9 accelerates the progression of hepatocellular carcinoma through the miR-149/CCND2 axis
Introduction
Hepatocellular carcinoma (HCC) is one of the most aggressive carcinomas and causes a great number of deaths each year worldwide (1,2). High malignancy of HCC is the primary cause of therapeutic failure and leads to poor prognosis (3,4). Although advances have been made in HCC therapies including surgery, targeted drug therapy, and immunotherapy, the therapeutic effect is still unsatisfactory, especially for patients with advanced HCC. Therefore, elucidating the underlying mechanisms of HCC progression is vital for developing novel therapies for HCC.
Circular RNAs (circRNAs) are non-coding RNAs with covalently linked 5' and 3' termini and have been demonstrated to exert vital functions in various biological processes (5,6). Intriguingly, one study has demonstrated that circRNAs exert key functions in regulating the progression of various human cancers (7). Pan et al. reported that the expression of circRNA MMP9 (circMMP9) was elevated in osteosarcoma, and knockdown of circMMP9 restrained the proliferation, migration, and invasion of osteosarcoma cells (8). Moreover, circMMP9 strengthens the migratory and invasive capacities of glioblastoma multiforme cells (9). The circRNAs can be developed as diagnostic and prognostic markers and therapeutic targets for HCC (10-12); however, the function of circMMP9 and related molecular mechanisms in HCC are unknown.
MicroRNAs (miRNAs) are small non-coding RNAs of approximately 22 nt in length and work as key regulators in gene expression (13). It has been well accepted that circRNAs regulate cancer progression via acting as miRNA sponges and reducing the expression of downstream targets (14,15). The circMMP9 promotes the progression of osteosarcoma and glioblastoma multiforme by sponging miR-1265 and miR-124, respectively (8,9). Importantly, miRNAs serve as important regulators in various human carcinomas (16,17). It has been reported that miR-149 is dysregulated in various carcinomas (18). The expression of miR-149 is downregulated in breast cancer tissues, and its suppression accelerates the progression of breast cancer (19). Xu et al. demonstrated that miR-149 weakens the migration and invasion of colorectal cancer cells via targeting FOXM1 (20). Additionally, miR-149 attenuates the metastasis of HCC via sponging PPM1F (21). Considering the important roles of miR-149 in various cancers including HCC, more studies are required to elucidate the function of miR-149 in HCC and evaluate the potential interaction between circMMP9 and miR-149 in HCC.
As a member of the cyclin family, cyclin D2 (CCND2) is a key regulator in cell cycle progression and proliferation (22), and it has been implicated in liver regeneration (23). In addition, the expression of CCND2 is inhibited in early recurrent HCC, and methylated CCND2 in the serum could be a prognostic indicator of HCC (24). Besides, our preliminary data suggested that miR-149 might bind to circMMP9 and CCND2. Therefore, the circMMP9/miR-149/CCND2 axis may be implicated in the progression of HCC. Herein, we hypothesized that circMMP9 may promote HCC progression through targeting miR-149 and subsequently regulating CCND2 expression. The knowledge of the pathogenesis of hepatocellular carcinoma (HCC) is limited. Surgery or transplantation are effective treatments, but recurrence and metastasis rates remain high, and 5-year survival rates are low (25). Many circRNAs are involved in the processes of HCC cell proliferation, invasion, and migration. As a result, circRNA may be a useful biomarker in the clinical diagnosis and treatment of HCC. To our knowledge, this is the first report of the role circMMP9 in HCC and it provides potential therapeutic targets for HCC. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-677/rc).
Methods
Patients
Tumor and adjacent normal (NC) tissues were obtained from 70 HCC patients at Mengchao Hepatobiliary Hospital of Fujian Medical University. The tissues were frozen and stored at −80 ℃ for analysis of the expression of circMMP9. The overall survival (OS) of these patients was monitored. Participant clinical pathological characteristics are shown in Table 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Review Board of Mengchao Hepatobiliary Hospital of Fujian Medical University (No. 2020-035-01) and informed consent was taken from all the patients.
Table 1
| Characteristics | All cases (n=70) | CircMMP9 expression | P value | |
|---|---|---|---|---|
| High (n=35) | Low (n=35) | |||
| Gender | 0.212 | |||
| Male | 45 | 20 | 25 | |
| Female | 25 | 15 | 10 | |
| Age (years) | 0.467 | |||
| <60 | 29 | 13 | 16 | |
| ≥60 | 41 | 22 | 19 | |
| Tumor size (cm) | 0.460 | |||
| <5 | 27 | 15 | 12 | |
| ≥5 | 43 | 20 | 23 | |
| Differentiation | 0.016* | |||
| Well/moderate | 30 | 10 | 20 | |
| Poor | 40 | 25 | 15 | |
| HBsAg | 0.803 | |||
| Positive | 45 | 22 | 23 | |
| Negative | 25 | 13 | 12 | |
| TNM stages | 0.030* | |||
| I/II | 39 | 15 | 24 | |
| III/IV | 31 | 20 | 11 | |
Data were analyzed by chi-squared test. *P<0.05. CircMMP9, circular RNA MMP9; HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; TNM, tumor-node-metastasis.
Cell culture and transfection
The Cell Bank of the Chinese Academy of Sciences (Shanghai, China) provided normal human hepatic cells L02 and HCC cells Huh7, HepG2, HCCLM3, and Hep3B. All of these cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Logan, UT, USA) with 12% fetal bovine serum (FBS; HyClone). To establish stable circMMP9-silencing cells, short hairpin RNAs (shRNAs) against circMMP9 (sh-circMMP9#1, #2, and #3) were cloned into the pLKO.1 lentiviral vector and lentiviral particles were generated. As a negative control, an empty lentiviral vector (sh-NC) was used. Then, Huh7 and Hep3B cells were infected with lentiviral particles for stable expression of sh-circMMP9. The coding region of CCND2 was inserted into the pcDNA3.1 vector. The Huh7 and Hep3B cells were transfected with the CCND2 overexpressing vector using Lipofectamine 3000 reagents (Thermo Fisher Scientific, Waltham, MA, USA). Cells were transfected with miR-149 mimics, inhibitor or negative controls (mimics and inhibitor NC) from RiboBio (Guangzhou, China) using Lipofectamine RNAiMAX (Thermo Fisher). The sequence of reagents used in cell transfection is listed in Table 2.
Table 2
| Name | Sequence (5'-3') |
|---|---|
| MiR-149 mimics | UCUGGCUCCGUGUCUUCACUCCC |
| Mimics NC | UCGCUUGGUGCAGGUCGGGAA |
| MiR-149 inhibitor | GGGAGTGAAGACACGGAGCCAGA |
| Inhibitor NC | CAGUACUUUUGUGUAGUACAA |
| sh-circMMP9#1 | GTGGAGGCGCAGATGGTGGAT |
| sh-circMMP9#2 | AGGAGTGGAGGCGCAGATGGT |
| sh-circMMP9#3 | GGGAGGAGTGGAGGCGCAGAT |
| sh-NC | UAAGGCUAUGAAGAGAUAC |
CircMMP9, circular RNA MMP9; NC, adjacent normal; sh, short hairpin.
Real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
The RNA was isolated from tumor and NC tissues from HCC patients and cells using TRIzolTM LS Reagent (Thermo Fisher) followed by RNA quantification. The RNA was subsequently reversely transcribed into complementary DNA (cDNA). Then, miRNA was isolated using mirPremier miRNA Isolation Kit (Merck, St. Louis, MO, USA) and reversely transcribed into cDNA with the miScript kit from QIAGEN (Germantown, MD, USA). We then quantified circMMP9 and miR-149 by real-time PCR and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or U6 snRNA. The PCR reaction system (20 µL) was as follows: 2 µL of cDNA template, 7.5 µL of ddH2O, 0.5 µL of paired primers (final concentration: 0.3 µM), and 10 µL of SYBR green. The PCR condition was as follows: initial denaturation at 94 ℃ for 3 min; 35 cycles of denaturation at 94 ℃ for 15 s, annealing at 60 ℃ for 30 s and elongation at 72 ℃ for 15 s; and final elongation at 72 ℃ for 3 min. The 2−∆∆Ct method was used for analyzing gene expression. Primers are shown in Table 3.
Table 3
| Name | Sequence (5'-3') |
|---|---|
| CircMMP9 | 5'-CCAGTTACAAGTTTAGGGCTGT-3' |
| 5'-TGTCTCCATTTGCTTCTTCTTCA-3' | |
| MiR-149 | 5'-CATCCTTTCTGGCTCCGTGT-3' |
| 5'-GCGTGATTCGTGCTCGTATATC-3' | |
| U6 snRNA | 5'-CTCGCTTCGGCAGCACA-3' |
| 5'-AACGCTTCACGAATTTGCGT-3' | |
| GAPDH | 5'-TGCACCACCAACTGCTTAGC-3' |
| 5'-GGCATGGACTGTGGTCATGAG-3' |
qRT-PCR, quantitative reverse-transcription polymerase chain reaction; CircMMP9, circular RNA MMP9.
RNase R digestion
Total RNA was isolated from Hep3B and HuH7 cells. We used RNase R (4 U/µg, Sigma-Aldrich, St. Louis, MO, USA) for digesting total RNA (5 µg) at 37 ℃ for 20 min. The RNA was then purified. Both circMMP9 and GAPDH were examined by qRT-PCR.
A subcutaneous HCC xenograft mouse model
The BALB/c nude mice (10-week-old, male) were provided by the Animal Center of Fujian Medical University. The subcutaneous HCC xenograft mouse model was established as previously described (26). The Huh7 cells were lentivirally transfected with sh-NC or sh-circMMP9, and 2×106 cells were subcutaneously injected into the mice’s left flanks. On days 7, 14, 21, 28, and 35, the tumor size was measured. Tumor volume was calculated using the formula length width width2/2. Mice were euthanized by adjusting CO2 flow into cages at 3 L/min until death, and tumors were excised and weighed. All animal procedures were approved by the Animal Care and Use Committee of Fujian Medical University (No. 2019009), in compliance with the institutional guidelines for the care and use of animals.
Luciferase activity
Wild-type (WT) or mutant (MUT) binding sites for miR-149 in circMMP9 and the CCND2 3' untranslated region (3' UTR) was inserted into the pmirGLO vector (Promega, Madison, WI, USA). Huh7 and Hep3B cells were co-transfected with the luciferase reporter circMMP9 or CCND2 as well as miR-149 mimics. We used NC mimics as a control. At 48 h post-transfection, cells were collected, and the Dual-Glo system (Promega) was used to examine the luciferase activity.
Cell Counting Kit-8 (CCK-8) assay
Transfection of Huh7 and Hep3B cells with sh-circMMP9, sh-circMMP9 + miR-149 inhibitor (anti-miR-149) or sh-circMMP9 + CCND2 overexpressing vector (CCND2) and seeded in 96-well plates. We used sh-NC as a control. At 24, 48, and 72 h, culture media were replaced. The CCK-8 from Dojindo (Rockville, MD, USA) was added and incubated for 4 h before measuring absorbance reading at 450 nm.
Cell cycle and apoptosis
Cell cycle was examined using FxCycleTM propidium iodide (PI)/RNase Staining Solution (Thermo Fisher) according to the manual. Cells were fixed in 75% ethanol solution and PI/RNAse solution was added into cells. Subsequently, cells were incubated for 30 min protected from light. The cell apoptosis kit was bought from Solarbio (Beijing, China). Cells were suspended and stained with FITC-Annexin V and PI for 20 min. Cell cycle and apoptosis were examined using a flow cytometer [Becton, Dickinson, and Co. (BD), Franklin Lakes, NJ, USA].
Transwell assay
Cell invasion was evaluated through the transwell assay. We seeded 1×105 Huh7 and Hep3B cells into the upper chamber precoated with Matrigel (BD) and incubated them for 24 h. Subsequently, invasive cells in the lower chamber were stained with crystal violet (Sigma) and imaged with a BX51 microscope (Olympus, Tokyo, Japan). Transwell chambers (8 µm-pore) were obtained from Corning (Corning, NY, USA).
Wound healing assay
Cell migration was evaluated by the wound healing assay. The Huh7 and Hep3B cells were seeded and grown to form a confluent monolayer. After removing the culture medium, the cell monolayer was scraped with cell combs (EMD Millipore, Darmstadt, Germany). Cells were then cultured for additional 24 h and observed with a BX51 microscope (Olympus), which was quantified using the Image J software (https://imagej.nih.gov/ij/download.html).
RNA immunoprecipitation (RIP) assay
The Huh7 and Hep3B cells were lysed, and supernatants were harvested after centrifugation. An Ago-2 antibody or normal IgG isotype was coated on magnetic beads and added into supernatants. Samples were incubated with gentle rotation overnight. The RNA was recovered using TRIzolTM LS Reagent (Thermo Fisher), and the enrichment of circMMP9 and miR-149 were examined by qRT-PCR.
RNA pull-down
Thermo Fisher provided a magnetic RNA-protein pull-down kit, which was used to investigate the interaction between circMMP9 and miR-149. The Huh7 and Hep3B cells were lysed. Subsequently, supernatants were harvested, and biotin-labeled miR-149 probes were added. After incubation for 5 h at 4 ℃, magnetic beads conjugated with streptavidin were added and incubated for an additional 2h.
Western blot
The Huh7 and Hep3B cells were transfected with miR-149 mimics, mimics NC, miR-149 inhibitor, inhibitor NC, sh-NC, sh-circMMP9, and sh-circMMP9 + anti-miR-149 or sh-circMMP9 + CCND2, respectively. Cells were then lysed, and cell lysates were obtained. Protein was quantified using the BCA kit from Santa Cruz (Dallas, TX, USA). Protein was electrophoresed and transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked and incubated with a CCND2 antibody (1:1,000, Abcam, Cambridge, UK). Following that, the membranes were washed and incubated with a secondary antibody conjugated to horseradish peroxidase (HRP) (Santa Cruz). To observe the bands, the ECL substrate (Bio-Rad) was used.
Statistical analysis
In this study, results were gathered from three independent assays and presented as mean standard deviation. The Student’s t-test and one-way analysis of variance (ANOVA) were used to determine the significance of variance in two and multiple groups, respectively. The Bonferroni post hoc test was used to compare two groups in multiple groups. The Kaplan-Meier plotter was used to compare the survival of patients with high and low circMMP9 expression. *P<0.05, **P<0.01 and ***P<0.001. A P value <0.05 was considered statistically significant.
Results
Elevated expression of circMMP9 was associated with the poor prognosis of patients with HCC
We collected tumor and NC tissues from HCC patients and analyzed the expression of circMMP9 to investigate the function of circMMP9 in HCC. CircMMP9 expression was significantly increased in tumor tissues (Figure 1A). Participants were divided into two groups based on the median expression of circMMP9 in tumor tissues: circMMP9high and circMMP9low. The survival of circMMP9high patients was obviously less favorable than that of circMMP9low patients (Figure 1B), suggesting that increased expression of circMMP9 was associated with the poor prognosis of HCC patients. The expression of circMMP9 was markedly correlated with tumor differentiation and tumor-nodes-metastases (TNM) stage rather than gender, age, tumor size, and hepatitis B surface antigen (HBsAg) (Table 1). We also found the expression of circMMP9 was significantly increased in HCC cells including Huh7, HepG2, HCCLM3, and Hep3B compared to that in L02 cells (Figure 1C). As Huh7 and Hep3B cells showed highest expression of circMMP9, they were selected for subsequent assays. As circRNAs are resistant to RNase R digestion, total RNA from Huh7 and Hep3B cells were treated with RNase R or mock (27). The qRT-PCR analysis showed that circMMP9, but not GAPDH, was resistant to RNase R digestion (Figure 1D), indicating that circMMP9 was a circRNA.

Knockdown of circMMP9 reduced HCC cell proliferation, induced cell cycle arrest and apoptosis in vitro and attenuated tumor growth in vivo
The circMMP9 was knocked down in Huh7 and Hep3B cells by lentiviral transduce of sh-circMMP9#1, #2, and #3. The expression of circMMP9 was efficiently suppressed in Huh7 and Hep3B cells, and the lowest expression of circMMP9 was observed in cells transfected with sh-circMMP#1 (Figure 2A). Therefore, sh-circMMP9#1 was used for subsequent knockdown of circMMP9. Knockdown of circMMP9 reduced Huh7 and Hep3B cell proliferation (Figure 2B). The Huh7 and Hep3B cells at G0/G1 stage were increased and cells at S stage were reduced by knockdown of circMMP9 (Figure 2C), suggesting that knockdown of circMMP9 caused cell cycle arresting at G0/G1 stage. Besides, apoptotic cell rate was obviously elevated in HCC cells by knockdown of circMMP9 (Figure 2D). Then, Huh7 cells transfected with sh-NC or sh-circMMP9 were subcutaneously injected into mice for evaluating tumor growth in vivo. Tumor volume and weight formed by Huh7 cells were dramatically suppressed by knockdown of circMMP9 (Figure 2E,2F). These data demonstrated that knockdown of circMMP9 attenuated tumor growth in vivo.

Knockdown of circMMP9 inhibited HCC cell migration and invasion
Wound healing and transwell assays were performed to study whether knockdown of circMMP9 affects HCC cell migration and invasion. Cell migration was notably reduced in cells with knockdown of circMMP9 compared to that of cells transfected with sh-NC (Figure 3A). Furthermore, cells with knockdown of circMMP9 exhibited lower invasive capacity than control cells (Figure 3B). Collectively, knockdown of circMMP9 suppressed HCC cell migration and invasion.

CircMMP9 sponged miR-149 in HCC cells
Nuclear and cytoplasmic fractions of Huh7 and Hep3B cells were separated, and the enrichment of circMMP9, U6, and GAPDH were evaluated using qRT-PCR. Same as GAPDH, circMMP9 mainly localized in the cytoplasm (Figure 4A). As circRNAs act as sponges for miRNAs, we predicted that miR-149 might be a novel target of circMMP9 using CircInteractome (Figure 4B) (28). The WT circMMP9 reporter’s luciferase activity, but not the MUT circMMP9 reporter, was inhibited by overexpression of miR-149 (Figure 4C). In addition, compared to the control probe, the miR-149 probe efficiently enriched circMMP9 from Huh7 and Hep3B cell lysates (Figure 4D). The RIP assays showed that both circMMP9 and miR-149 were enriched in the anti-Ago2-immunoprecipitated complex (Figure 4E). It was concluded that circMMP9 could sponge miR-149 in HCC cells.

MiR-149 targeted CCND2 to reduce its expression in HCC cells
StarBase v2.0 (https://bio.tools/starbase) was used to find downstream targets of miR-149 in HCC, and we discovered a potential miR-149 binding site in the 3' UTR of CCND2 (Figure 5A). The luciferase assay revealed that overexpression of miR-149 inhibited the luciferase activity of the WT CCND2 reporter, but not after the binding site was mutated (Figure 5B). The expression of CCND2 was reduced in miR-149 overexpressing cells compared to that in cells transfected with mimics NC (Figure 5C). The miR-149 was knocked down by transfection of miR-149 inhibitor in Huh7 and Hep3B cells (Figure 5D), and the expression of CCND2 was elevated by knockdown of miR-149 (Figure 5E). Taken together, miR-149 targeted CCND2 to suppress its expression in HCC cells.

Knockdown of circMMP9 attenuated HCC malignant phenotypes through the miR-149/CCND2 axis
To investigate whether knockdown of circMMP9-mediated alleviation of HCC progression was dependent on the miR-149/CCND2 axis, Huh7 and Hep3B cells were transfected with sh-circMMP9 and an inhibitor of miR-149 or the CCND2 overexpressing vector. The expression of CCND2 was decreased in Huh7 and Hep3B cells with knockdown of circMMP9, but it was reversed by concomitant suppression of miR-149 or overexpression of CCND2 (Figure 6A). Knockdown of circMMP9-mediated inhibition of HCC cell proliferation was abrogated by silencing of miR-149 or overexpression of CCND2 (Figure 6B). Additionally, knockdown of circMMP9-induced cell cycle arrest and apoptosis were reversed by concomitant suppression of miR-149 or overexpression of CCND2 in Huh7 and Hep3B cells (Figure 6C,6D). Both cell migration and invasion of HCC were mitigated by knockdown of circMMP9, which was abolished by concomitant suppression of miR-149 or overexpression of CCND2 (Figure 6E-6G). These data indicated that knockdown of circMMP9 attenuated HCC progression through the miR-149/CCND2 axis.

Discussion
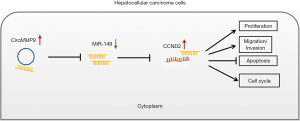
The malignancy of HCC is a highly lethal hepatic carcinoma and the second leading cause of cancer-related death globally, posing a severe health threat and huge economic pressures (29,30). Elucidating the regulatory mechanism of HCC progression is vital for improving the prognosis and developing novel therapies for HCC. CircRNA has distinct structural and biological properties and is no longer thought to be a byproduct of the splicing process. It has important pathological and physiological roles in various tumors and plays an important regulatory role in them. According to one study, circRNAs act as miRNA adsorption sponges to counteract miRNA-mediated inhibition of mRNA expression (31). The role of the circRNA/miRNA/mRNA regulatory axis in the development and metastasis of HCC is gradually becoming clear (32,33). Previous research has found that a large number of ncRNAs, including circRNAs and miRNAs, are dysregulated in HCC (34-36). In present study, we reported that circMMP9 sponges miR-149 and enhances the expression of CCND2, thus accelerating the progression of HCC for the first time (Figure 7). We provide an experimental and theoretical foundation for HCC targeted therapy using circRNAs/miRNA/mRNA networks.
Increasing evidence has shown that circRNAs are implicated in various human carcinomas including HCC (37-39). The current study of circRNAs faces the following challenges: (I) most current research on circRNA mechanisms has focused on miRNAs acting through the circRNA/miRNA/mRNA regulatory axis, with few reports on other mechanisms. CircRNAs, on the other hand, are translatable, resulting in the production of specific proteins. As a new field in life sciences, encoding cyclic RNAs may become a potential therapeutic target for liver cancer. (II) At the moment, most studies focus on circRNAs and their regulated downstream target genes, with few reports on the upstream regulators that regulate circRNAs. For instance, Huang et al. found that circRNA_104348 promoted the proliferation, migration, and invasion of HCC cells and inhibited HCC cell apoptosis via targeting miR-187-3p and triggering Wnt/β-catenin signaling (40). The circMTO1 attenuates the progression of HCC through sponging miR-9 and enhancing the expression of p21 (41). Intriguingly, circMMP9 is upregulated in various cancers such as glioblastoma multiforme and osteosarcoma and acts as an oncogene to promote their progression (8,9). In consistence, we firstly reported that circMMP9 is highly expressed in HCC and its high expression indicates the poor prognosis of patients with HCC. In addition, Pan et al. reported that knockdown of circMMP9 suppressed osteosarcoma cell proliferation, migration, and invasion and induced its apoptosis (8). Therefore, we examined whether knockdown of circMMP9 exhibits an anti-tumor activity in HCC. We observed that knockdown of circMMP9 suppressed malignant phenotypes including proliferation, migration, and invasion of HCC cells. Importantly, knockdown of circMMP9 restrained HCC growth in vivo. Consistent with previous studies, our study supports the notion that circMMP9 works as an oncogene to enhance cancer progression.
The competitive endogenous RNA (ceRNA) hypothesis has been proposed to describe the crosstalk among non-coding RNAs, which titrates out miRNAs to regulate the expression of downstream targets (42). The circRNAs can function as ceRNAs to target miRNAs and regulate the expression of target genes in cancers (43). The circMMP9 has been demonstrated to exert its oncogenic activity through acting as sponges for miRNAs including miR-149, miR-1265, and miR-124 (8,9,44). Consistently, we observed that circMMP9 sponged miR-149 to reduce its expression in HCC cells, identifying miR-149 as a novel target of circMMP9 in HCC. Mounting evidence supports that miR-149 functions as a tumor suppressor in several cancers, including HCC (45-47). We found that silencing of miR-149 reversed circMMP9 knockdown-mediated suppression of HCC progression, supporting the notion that miR-149 is a tumor suppressor.
The miRNAs works as guides via base-pairing with downstream target mRNAs to negatively regulate their expression (45). Various downstream targets of miR-149 have been identified, such as PPM1F, PARP-2, FOXM1, and Rap1 (46). In this study, CCND2 was identified as novel downstream target of miR-149. The miR-149 binds to the 3' UTR of CCND2 to reduce its expression in HCC. Further, CCND2 has been proposed to serve as a key regulator in the modulation of cell cycle and proliferation in various cancers (47-49). Consistently, we observed that overexpression of CCND2 facilitated HCC cell cycle and proliferation. However, further studies were required to explore miR-149-mediated regulation of CCND2 in detail and downstream signaling of CCND2 in the progression of HCC.
To summarize, we found that circMMP9 accelerates the progression of HCC by targeting the miR-149/CCND2 axis for the first time. Our investigation not only implies a novel regulatory mechanism of HCC progression, but also shows the potency of circMMP9/miR-149/CCND2 axis to be developed as diagnostic and prognostic markers and therapeutic targets for HCC. However, additional research is required to fully understand the nature of the regulation and more samples from patients with HCC and animal models of HCC should be adopted in future studies to evaluate the roles of the circMMP9/miR-149/CCND2 axis in HCC for clinical application. In addition, we only identified the circMMP9/miR-149/CCND2 axis in this study. As a circRNA generally has several target miRNAs and a miRNA also targets various genes, studies are ongoing to explore other potential downstream miRNAs and genes of circMMP9 in HCC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-677/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-677/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-677/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Review Board of Mengchao Hepatobiliary Hospital of Fujian Medical University (No. 2020-035-01) and informed consent was taken from all the patients. All animal procedures were approved by the Animal Care and Use Committee of Fujian Medical University (No. 2019009), in compliance with the institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Lee WC, Jeng LB, Chen MF. Estimation of prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg 2002;89:311-6. [Crossref] [PubMed]
- Ren Z, Ma X, Duan Z, et al. Diagnosis, Therapy, and Prognosis for Hepatocellular Carcinoma. Anal Cell Pathol (Amst) 2020;2020:8157406. [Crossref] [PubMed]
- Greene J, Baird AM, Brady L, et al. Circular RNAs: Biogenesis, Function and Role in Human Diseases. Front Mol Biosci 2017;4:38. [Crossref] [PubMed]
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013;495:333-8. [Crossref] [PubMed]
- Kristensen LS, Hansen TB, Venø MT, et al. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene 2018;37:555-65. [Crossref] [PubMed]
- Pan G, Hu T, Chen X, et al. Upregulation Of circMMP9 Promotes Osteosarcoma Progression Via Targeting miR-1265/CHI3L1 Axis. Cancer Manag Res 2019;11:9225-31. [Crossref] [PubMed]
- Wang R, Zhang S, Chen X, et al. EIF4A3-induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol Cancer 2018;17:166. [Crossref] [PubMed]
- Qiu L, Xu H, Ji M, et al. Circular RNAs in hepatocellular carcinoma: Biomarkers, functions and mechanisms. Life Sci 2019;231:116660. [Crossref] [PubMed]
- Lv S, Li Y, Ning H, et al. CircRNA GFRA1 promotes hepatocellular carcinoma progression by modulating the miR-498/NAP1L3 axis. Sci Rep 2021;11:386. [Crossref] [PubMed]
- He S, Yang J, Jiang S, et al. Circular RNA circ_0000517 regulates hepatocellular carcinoma development via miR-326/IGF1R axis. Cancer Cell Int 2020;20:404. [Crossref] [PubMed]
- Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 2007;23:175-205. [Crossref] [PubMed]
- Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol 2016;238:42-51. [Crossref] [PubMed]
- Lu Q, Liu T, Feng H, et al. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Mol Cancer 2019;18:111. [Crossref] [PubMed]
- Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol 2009;4:199-227. [Crossref] [PubMed]
- Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 2014;20:460-9. [Crossref] [PubMed]
- He Y, Yu D, Zhu L, et al. miR-149 in Human Cancer: A Systemic Review. J Cancer 2018;9:375-88. [Crossref] [PubMed]
- Sánchez-González I, Bobien A, Molnar C, et al. miR-149 Suppresses Breast Cancer Metastasis by Blocking Paracrine Interactions with Macrophages. Cancer Res 2020;80:1330-41. [Crossref] [PubMed]
- Xu K, Liu X, Mao X, et al. MicroRNA-149 suppresses colorectal cancer cell migration and invasion by directly targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem 2015;35:499-515. [Crossref] [PubMed]
- Luo G, Chao YL, Tang B, et al. miR-149 represses metastasis of hepatocellular carcinoma by targeting actin-regulatory proteins PPM1F. Oncotarget 2015;6:37808-23. [Crossref] [PubMed]
- Ando K, Ajchenbaum-Cymbalista F, Griffin JD. Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci U S A 1993;90:9571-5. [Crossref] [PubMed]
- Zhou J, Ju WQ, Yuan XP, et al. miR-26a regulates mouse hepatocyte proliferation via directly targeting the 3' untranslated region of CCND2 and CCNE2. Hepatobiliary Pancreat Dis Int 2016;15:65-72. [Crossref] [PubMed]
- Tsutsui M, Iizuka N, Moribe T, et al. Methylated cyclin D2 gene circulating in the blood as a prognosis predictor of hepatocellular carcinoma. Clin Chim Acta 2010;411:516-20. [Crossref] [PubMed]
- Wang M, Gu B, Yao G, et al. Circular RNA Expression Profiles and the Pro-tumorigenic Function of CircRNA_10156 in Hepatitis B Virus-Related Liver Cancer. Int J Med Sci 2020;17:1351-65. [Crossref] [PubMed]
- Schmidt KM, Geissler EK, Lang SA. Subcutaneous Murine Xenograft Models: A Critical Tool for Studying Human Tumor Growth and Angiogenesis In Vivo. Methods Mol Biol 2016;1464:129-37. [Crossref] [PubMed]
- Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development 2016;143:1838-47. [Crossref] [PubMed]
- Dudekula DB, Panda AC, Grammatikakis I, et al. CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol 2016;13:34-42. [Crossref] [PubMed]
- Asafo-Agyei KO, Samant H. Hepatocellular Carcinoma. In: StatPearls. Treasure Island: StatPearls Publishing, 2021.
- Brown ZJ, Yu SJ, Heinrich B, et al. Indoleamine 2,3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol Immunother 2018;67:1305-15. [Crossref] [PubMed]
- de la Peña M, Ceprián R, Cervera A. A Singular and Widespread Group of Mobile Genetic Elements: RNA Circles with Autocatalytic Ribozymes. Cells 2020;9:2555. [Crossref] [PubMed]
- Chen X, Ye Q, Chen Z, et al. Long non-coding RNA muskelin 1 antisense RNA as a potential therapeutic target in hepatocellular carcinoma treatment. Bioengineered 2022;13:12237-47. [Crossref] [PubMed]
- Lin X, Xiang X, Feng B, et al. Targeting Long Non-Coding RNAs in Hepatocellular Carcinoma: Progress and Prospects. Front Oncol 2021;11:670838. [Crossref] [PubMed]
- Chen Y, Yuan B, Wu Z, et al. Microarray profiling of circular RNAs and the potential regulatory role of hsa_circ_0071410 in the activated human hepatic stellate cell induced by irradiation. Gene 2017;629:35-42. [Crossref] [PubMed]
- Cui S, Qian Z, Chen Y, et al. Screening of up- and downregulation of circRNAs in HBV-related hepatocellular carcinoma by microarray. Oncol Lett 2018;15:423-32. [PubMed]
- Fu L, Yao T, Chen Q, et al. Screening differential circular RNA expression profiles reveals hsa_circ_0004018 is associated with hepatocellular carcinoma. Oncotarget 2017;8:58405-16. [Crossref] [PubMed]
- Ng WL, Mohd Mohidin TB, Shukla K. Functional role of circular RNAs in cancer development and progression. RNA Biol 2018;15:995-1005. [Crossref] [PubMed]
- Liu H, Lan T, Li H, et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics 2021;11:1396-411. [Crossref] [PubMed]
- Yin Y, Long J, He Q, et al. Emerging roles of circRNA in formation and progression of cancer. J Cancer 2019;10:5015-21. [Crossref] [PubMed]
- Huang G, Liang M, Liu H, et al. CircRNA hsa_circRNA_104348 promotes hepatocellular carcinoma progression through modulating miR-187-3p/RTKN2 axis and activating Wnt/β-catenin pathway. Cell Death Dis 2020;11:1065. [Crossref] [PubMed]
- Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology 2017;66:1151-64. [Crossref] [PubMed]
- Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 2011;146:353-8. [Crossref] [PubMed]
- Verduci L, Strano S, Yarden Y, et al. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol 2019;13:669-80. [Crossref] [PubMed]
- Xia B, Hong T, He X, et al. A circular RNA derived from MMP9 facilitates oral squamous cell carcinoma metastasis through regulation of MMP9 mRNA stability. Cell Transplant 2019;28:1614-23. [Crossref] [PubMed]
- Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics 2010;11:537-61. [Crossref] [PubMed]
- Xu Y, Chen X, Lin L, et al. MicroRNA-149 is associated with clinical outcome in human neuroblastoma and modulates cancer cell proliferation through Rap1 independent of MYCN amplification. Biochimie 2017;139:1-8. [Crossref] [PubMed]
- Park SY, Lee CJ, Choi JH, et al. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res 2019;38:399. [Crossref] [PubMed]
- Ding ZY, Li R, Zhang QJ, et al. Prognostic role of cyclin D2/D3 in multiple human malignant neoplasms: A systematic review and meta-analysis. Cancer Med 2019;8:2717-29. [Crossref] [PubMed]
- Chang L, Guo R, Yuan Z, et al. LncRNA HOTAIR Regulates CCND1 and CCND2 Expression by Sponging miR-206 in Ovarian Cancer. Cell Physiol Biochem 2018;49:1289-303. [Crossref] [PubMed]
(English Language Editor: J. Jones)