Bioinformatic-based mechanism identification of E2F1-related ceRNA and E2F1 immunoassays in hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) accounts for about 80% of primary liver cancer cases (1). The major pathogenic causes of HCC include hepatitis [e.g., hepatitis B virus (HBV) and hepatitis C virus (HCV)] alcoholism, smoking, obesity, and congenital inheritance. In the United States, the latest cancer statistics showed that the number of deaths from liver cancer reached 30,230 in 2021 (2). HCV infection is the leading cause of liver cancer in Western countries and causes approximately 1/4 of all the HCC cases. In developing countries (e.g., China), HBV infection is the predominant cause of liver cancer (3). Compared to other cancers, the prognosis of liver cancer is still relatively poor, and it has a 5-year survival rate of only 20% (4). Thus, research urgently needs to be conducted to identify effective biological targets related to liver cancer, especially HCC.
Most long non-coding RNAs (lncRNAs) do not encode proteins (5), and have even been considered junk DNA; however, in-depth research on non-coding RNAs have revealed that many lncRNAs regulate gene expression during or after transcriptional processes. LncRNAs affect a series of pathological and physiological processes by participating in the biological regulation, such as chromosome imprinting, epigenetic regulation, cell proliferation and cell cycle (6,7). Under the recently proposed potential competing endogenous RNA (ceRNA) theory, lncRNA competes to occupy a large number of micro RNAs (miRNAs) in the cell and acts like a sponge to buffer and interfere with the protein encoded by the target gene messenger RNA (mRNA) (8). This kind of mechanism also provides a good entry point for researchers to explore the mechanism of tumorigenesis and development and find effective tumor therapy targets.
E2F1 is an important transcription factor involved in multiple steps, including DNA damage response and cell-cycle regulation (9). Previous studies have shown that the overexpression of E2F1 is closely related to the occurrence and development of various malignant tumors, including HCC (10-12). The abnormal activation of E2F1 affects its downstream transcriptional targets, resulting in DNA replication stress (13). The above mechanisms play an important role in the occurrence and development of liver cancer. At present, there is still a lot of room for exploration on the regulatory mechanism upstream of E2F1. By finding out the effective regulatory mechanism related to E2F1, and then inhibiting the expression of E2F1, it is helpful to finally achieve the purpose of inhibiting the development of HCC.As our current understanding of E2F1 is insufficient, we sought to study the mechanism and related regulation of E2F1 in HCC. Thus, based on bioinformatics, we adopted the ceRNA mechanism as an entry point to analyze the lncRNAs and miRNAs related to E2F1 and explore the correlation between E2F1 and immune infiltration levels of various types of immune cells in HCC to identify potential biological targets and to prepare for subsequent basic research. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-674/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research is divided into the following parts. First, we preliminarily verified that E2F1 is significantly overexpressed in malignant tumors including HCC through the TCGA database. Kaplan-Meier analysis was used to discuss the relationship between E2F1 expression and survival. Second, a retrospective analysis of 364 patients was performed to explore the relationship between E2F1 and clinicopathological parameters. The specificity and sensitivity of E2F1 as a prognostic indicator were also evaluated. Second, a retrospective analysis of 364 patients was performed to explore the relationship between E2F1 and clinicopathological parameters. The specificity and sensitivity of E2F1 as a prognostic indicator were also evaluated. Third, bioinformatics analysis was used to explore potential ceRNA mechanisms, and a total of 6 potential E2F1-related signaling pathways were screened. Then, the relationship between E2F1 and tumor-related immunity was explored using the Tumor IMmune Estimation Resource (TIMER) database. Finally, the drug sensitivity of E2F1 as a therapeutic target in HCC was explored.
Differential expression and survival analyses of E2F1
HCC-related transcriptome data and patient clinical information downloaded from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). A differential expression map of E2F1 in pan-cancer was obtained from the TIMER2.0 database (http://timer.cistrome.org/). Based on the magnitude of the P value of E2F1 for different cancer types, the P value was divided into P<0.001, P<0.01, and P<0.05. The differential expression analysis of E2F1 in HCC was analyzed and plotted using the “limma”, “ggplot2,” and “ggpubr” packages. Prognosis-related survival curves were downloaded from the Gene Expression Profiling Interactive Analysis (GEPIA) database (http://gepia.cancer-pku.cn).
Analysis of clinical prognostic factors
The correlation between E2F1 expression and each prognostic factor was analyzed and plotted using the “limma” and “ggpubr” packages. Heatmaps for each clinical prognostic factor were drawn using the “limma” and “ComplexHeatmap” packages. The receiver operator characteristic (ROC) curves, calibration curves, and nomograms were made using the “survival,” “survminer,” “timeROC,” “regplot,” and “rms” analysis packages. Univariate and multivariate Cox analyses were conducted, and forest plots were generated using the “survival” package.
Establishment of mRNA-miRNA-lncRNA co-expression network and survival analysis of miRNA and lncRNA
The mRNA-miRNA and miRNA-LncRNA interaction data were downloaded from the starBase database (http://starbase.sysu.edu.cn/), with a programNum ≥2 as one of the mRNA-miRNA screening criteria. The correlation coefficient values (an R value >0.2 was defined as a positive correlation, and an R value <–0.2 was defined as negative correlation), differential expression values (a P value <0.01 was considered statistically significant), and survival curve values (a P value <0.05 was considered statistically significant) were screened out and plotted using the R language package. A conceptual diagram of the potential ceRNA mechanisms associated with E2F1 was drawn with BioRender.
E2F1 immune correlation analysis
The correlation analyses between E2F1 and various immune cells and immune checkpoints were visualized using various R language packages, including “limma,” “reshape2,” “ggplot2,” “ggpubr,” “vioplot,” “ggExtra,” and “corrplot.” The p values were calculated using the Spearman statistical method. A positive correlation was defined as a P value <0.05, an R value >0.2, a negative correlation was defined as a P value <0.05, an R value <–0.2, and a P value >0.05 was defined as not significant. E2F1, PDCD1, CD274, CTLA4, and LAG3 were analyzed using the TIMER 2.0 database (http://timer.cistrome.org/).
E2F1-related drug sensitivity evaluation
half maximal inhibitory concentration (IC50) represents the concentration required for the 50% inhibition of drug concentration. We calculated the IC50 of drugs using the “pRRophetic” R package with its dependencies “car, ridge preprocessCore, genefilter, and sva,” which contained information on the effects of 138 drugs. Boxplots were drawn using the “ggplot2” R package. A P value <0.05 indicated a statistically significant difference.
Statistical analysis
Wilcoxon rank-sum test was used to compare the difference between the two groups. Differential expression data were analyzed by “DESeq2” and “survival” R software. KM survival analysis was used for ROC curve analysis, univariate and multivariate Cox regression analysis. Spearman’s test was used to measure correlations between E2F1 and immune functions. And P value <0.05 was regarded as the significant threshold.
Results
Differential expression and survival analyses of E2F1 in HCC
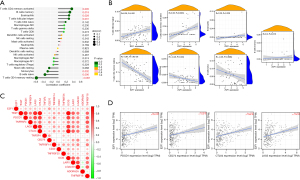
We analyzed the expression of E2F1 in 38 cancer types in the TIMER2.0 database and found that E2F1 was significantly differentially expressed between the tumor group and the normal group in terms of 20 malignant tumors, including HCC (P<0.001; see Figure 1A). We downloaded the HCC-related transcriptome data and patient clinical information downloaded from TCGA database, and found that E2F1 was significantly differentially expressed in malignant tumors (P<0.01; see Figures 1B,1C). We also searched E2F1-related disease-free survival (DFS) and overall survival (OS) of HCC patients in the GEPIA database and found that there were significant differences in the prognosis of the high-risk group (182 cases) and the low-risk group (182 cases) (DFS: P=0.0027, OS: P=0.0025), and the prognosis of the low-risk group was significantly better than that of the high-risk group in terms of both OS (see Figure 1D) and DFS (see Figure 1E).

Analysis of prognostic factors of E2F1 in HCC
Subsequently, we analyzed the key prognostic factors related to E2F1 and found that there was a significant difference between stage I, stage II, and stage III, and between stage III and IV (P<0.01; see Figure 2A). Significant differences were found among all the grades, except grade 3 and grade 4 (see Figure 2B). The expression of E2F1 differed significantly between T1 and T2, T3, and T4 (P<0.01; see Figure 2C), and there were significant differences in tumor (T) stage, stage, and grade in the high- and low-expression groups (P<0.001; see Figure 2D). In the sensitivity and specificity analyses, the ROC curve of the target gene E2F1 showed that the areas under the curve (AUCs) at 1, 3, and 5 years were 0.646, 0.628, and 0.584, respectively (see Figure 2E). A nomogram was drawn to assess whether E2F1 could predict survival time in HCC (see Figure 2F), and the feasibility of this prediction method was validated with a calibration curve (see Figure 2G). Finally, we concluded that E2F1 expression and HCC stage were independent risk factors for prognosis through univariate and multivariate Cox regression analyses (see Figure 2H,1I).

Establishment of mRNA-miRNA co-expression network and related miRNA survival analysis
After downloading the E2F1-miRNA interaction data from the starBase database and using the R language package for the analysis, we screened 2 groups of miRNAs that were co-expressed and negatively correlated with E2F1 (i.e., had a correlation coefficient <value –0.2, and a P value <0.001) in preparation for the subsequent screening of potential targets and pathways. The subsequent analysis showed that the 2 groups of miRNAs were differentially expressed in the normal group and the tumor group (P<0.001), and the above-mentioned miRNA-related prognosis survival curve analysis showed that the high-expression group had a better result than the low-expression group (P<0.05; see Figure 3A,3B).

Next, we downloaded the lncRNA data that interacted with miR-29b-3p and miR-29c-3p from the starBase database, and screened and analyzed the correlation coefficients (those with a correlation coefficient value >0.2), log fold change (FC) values (those with a log FC value >0), survival curves, and the differential expression between the tumor group and the normal group (P<0.01) by R language. We also selected lncRNAs whose expression levels were positively correlated with E2F1 according to the above screening results (those with a correlation coefficient value >0.2, and a P value <0.01). Finally, the miR-29b-3p-related lncRNAs (i.e., LINC01224, PCBP1-AS1, and ITGA9-AS1), and the miR-29c-3p-related lncRNAs (i.e., SNHG7, THUMPD3-AS1, and LINC02323) were screened (see Figure 4A,4B). Based on the above results, the possible potential ceRNA mechanism diagram for E2F1 was constructed (see Figure 5).


Correlation analyses of E2F1 with various immune cells and immune checkpoints
Additionally, we analyzed the correlations between immune cells and the levels of immune infiltration for E2F1, and found that cluster of differentiation (CD)4 memory activated T cells, memory B cells, eosinophils, and follicular helper T cells were positively correlated with E2F1 (R>0.2, P<0.01), and monocytes, naïve B cells, and CD4 memory resting T cells were negatively correlated with E2F1 (R<–0.2, P<0.01; see Figure 6A,6B). The TIMER database-related immune checkpoint analysis showed that E2F1 was positively correlated with PDCD1, CTLA4, and LAG3 (R>0.2, P<0.01; see Figure 6C,6D).
Drug sensitivity evaluation
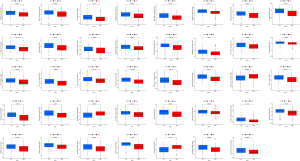
We examined the relationship between the risk score and the IC50 of various drugs used in the clinical treatment of HCC, including imatinib, etoposide, and paclitaxel. Patients in the high-expression group appeared to be more susceptible to most drugs than those in the low-expression group (P<0.001; see Figure 7).
Discussion
E2F1 was the first member of the E2F transcription factor family, which comprises 8 proteins, to be discovered (14). Based on their different functions, they are usually classified as activators (E2F1-e2f3a) or inhibitors (E2F3b-E2F8) (15). Studies have shown that E2F1 mainly regulates the transcription of S-phase cyclins and related genes required for DNA replication, DNA repair, and apoptosis (16). At present, the common genes that cause the abnormal activation of E2F1 mainly include retinoblastoma (Rb), Ras, and PI3K. The abnormal activation of E2F1 affects its downstream transcriptional targets, resulting in DNA replication stress (16). Its transcriptional targets include cyclin E and RRM2. Cyclin E promotes the phosphorylation of essential DNA replication factors to initiate and allow the progression of bidirectional DNA synthesis. Cyclin E overexpression results in enhanced CDK2 activity and cell cycle progression, thereby reducing the ability of cells to regulate the G1 (DNA prophase)-S (DNA replication period) transition (17). This regulatory mechanism has been widely observed in a number of malignancies (17-19). In addition, another important transcriptional target of E2F1 that could contribute to DNA replication stress is RRM2 (20), and the above signaling pathway of E2F1 has been reported in adrenocortical carcinoma (21), colorectal cancer (22), pancreas cancer (23), and other malignant tumors.
Using the TIMER database, we sought to identify the immune cells correlated with E2F1 in terms of the level of immune infiltration in HCC. We found that the expression of E2F1 was positively correlated with CD4 memory activated T cells, memory B cells, eosinophils, and follicular helper T cells, and negatively correlated with monocytes, naïve B cells, and CD4 memory resting T cells. Studies have shown that HTLV-1 basic leucine zipper factor (HBZ) is a related viral factor required for the viral replication and transformation of infected cells. HBZ protein interacts with the Rb/E2F-1 complex and induces the transcription of E2F target genes. The activation of the Rb/E2F pathway by the HBZ protein accelerates G1/S transition and apoptosis in primary CD4+ T cells (24). The downregulation of E2F1 decreases the susceptibility of CD8+ T cells. E2F1 has been shown to be a transcription factor for TBX21, a Th1 cell-specific transcription factor that controls the expression of the hallmark Th1 cytokine and interferon gamma (IFN-γ) (25). Thus, E2F1 plays an important role in tumor immunity by affecting the activation of effector CD8+ T cells (26).
E2F1 also significantly represses the transcriptional activity of the interleukin (IL)-6 promoter, while the overexpression of E2F1 promotes this activity. E2F1 regulates macrophage cytokine expression via IL-6 in nasopharyngeal carcinoma (NPC) cell supernatants, which supports its utility in the tumor microenvironment (TME). In a xenograft tumorigenesis model, small interfering–RNAs targeting E2F1 or E2F3 significantly inhibited tumor growth and reduced immune cell infiltration in the TME (27), which suggests that E2F1 can be regulated by modulating macrophage function. Further, E2F1 transactivates the IL-6 promoter, a very important inflammatory cytokine. However, E2F1 mostly acts as an inhibitor to negatively regulate dendritic cells (28), but its activation in mouse bone marrow-derived dendritic cells (DC2.4) cells is decreased by E2F1 knockdown and enhanced by E2F1 overexpression. The mechanism underlying this phenomenon is unclear; however, it may be related to the activation of p38 mitogen-activated protein kinase (MAPK) by E2F1, which directly promotes the activation of DC2.4 cells.
According to previous findings, the silencing of LINC01224 downregulates CHEK1 expression by competitively binding to miR-330-5p, thereby inhibiting HCC progression. Additionally, LINC01224 has been shown to induce HCC progression in vitro and accelerate HCC formation in nude mice by increasing CHEK1 expression (29). There are differences in the expression of PCBP1-AS1 in HCC. Notably, PCBP1-AS1 promotes HCC progression and HCC cell metastasis by combining with PCBP1 and regulating the PCBP1/PRL-3/serine/threonine kinase (AKT) pathway (30). The expression of lncRNA SNHG7 is upregulated in HCC, and elevated SNHG7 expression is closely associated to the staging, grading, vascular invasion, and poor prognosis in HCC patients. SNHG7 promotes HCC progression by regulating miR-122-5p and RPL4 (31). Additionally, studies have confirmed that low expression of miR-29b-3p, miR-29c-3p is associated with tumor growth, multiple pathological features, and shorter OS (32). Several HCC-related reports have noted that the overexpression of miR-29b-3p, miR-29c-3p significantly inhibits the proliferation, apoptosis, migration, and tumor growth of HCC cells in vivo (33,34).
In this study, using TCGA, GEPIA and starBase databases, we identified the miRNAs (i.e., miR-29b-3p and miR-29c-3p) related to transcription factor E2F1 in HCC by R language. We also used the lncRNAs related to E2F1 (i.e., miR-29b-3p, and miR-29c-3p) to construct ceRNA models. Further, we analyzed the related immune cell infiltration, immune checkpoints, and drug sensitivity of E2F1 using the TIMER database. It should be noted that this research was based on a bioinformatics analysis; thus, the validity of the findings needs to be further verified by basic experimental research. However, our results still provide a very valuable direction and reference for research on transcription factor E2F1, which may be helpful in identifying research targets for future HCC-related molecular biological therapy and immunotherapy.
Acknowledgments
Funding: This study was supported by the Fundamental Research Funds for the Provincial Universities (No. 2018-KYYWF-0534).
Footnote
Reporting Checklist: Both authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-674/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-674/coif). Both authors report this study was supported by the Fundamental Research Funds for the Provincial Universities (No. 2018-KYYWF-0534). Both authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844-55. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Matsumoto A, Pasut A, Matsumoto M, et al. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2017;541:228-32. [Crossref] [PubMed]
- Chan JJ, Tay Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int J Mol Sci 2018;19:1310. [Crossref] [PubMed]
- Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017;36:5661-7. [Crossref] [PubMed]
- Kopp F, Mendell JT. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018;172:393-407. [Crossref] [PubMed]
- González-Romero F, Mestre D, Aurrekoetxea I, et al. E2F1 and E2F2-Mediated Repression of CPT2 Establishes a Lipid-Rich Tumor-Promoting Environment. Cancer Res 2021;81:2874-87. [Crossref] [PubMed]
- Chen Q, Wang L, Jiang M, et al. E2F1 interactive with BRCA1 pathway induces HCC two different small molecule metabolism or cell cycle regulation via mitochondrion or CD4+T to cytosol. J Cell Physiol 2018;233:1213-21. [Crossref] [PubMed]
- Enjoji S, Yabe R, Tsuji S, et al. Stemness Is Enhanced in Gastric Cancer by a SET/PP2A/E2F1 Axis. Mol Cancer Res 2018;16:554-63. [Crossref] [PubMed]
- Rocca MS, Di Nisio A, Marchiori A, et al. Copy number variations of E2F1: a new genetic risk factor for testicular cancer. Endocr Relat Cancer 2017;24:119-25. [Crossref] [PubMed]
- Meng P, Ghosh R. Transcription addiction: can we garner the Yin and Yang functions of E2F1 for cancer therapy? Cell Death Dis 2014;5:e1360. [Crossref] [PubMed]
- Cao AR, Rabinovich R, Xu M, et al. Genome-wide analysis of transcription factor E2F1 mutant proteins reveals that N- and C-terminal protein interaction domains do not participate in targeting E2F1 to the human genome. J Biol Chem 2011;286:11985-96. [Crossref] [PubMed]
- Pützer BM, Engelmann D. E2F1 apoptosis counterattacked: evil strikes back. Trends Mol Med 2013;19:89-98. [Crossref] [PubMed]
- Fouad S, Hauton D, D'Angiolella V. E2F1: Cause and Consequence of DNA Replication Stress. Front Mol Biosci 2020;7:599332. [Crossref] [PubMed]
- Caruso JA, Duong MT, Carey JPW, et al. Low-Molecular-Weight Cyclin E in Human Cancer: Cellular Consequences and Opportunities for Targeted Therapies. Cancer Res 2018;78:5481-91. [Crossref] [PubMed]
- Huber AR, Tan D, Sun J, et al. High expression of carbonic anhydrase IX is significantly associated with glandular lesions in gastroesophageal junction and with tumorigenesis markers BMI1, MCM4 and MCM7. BMC Gastroenterol 2015;15:80. [Crossref] [PubMed]
- DeLair DF, Burke KA, Selenica P, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol 2017;243:230-41. [Crossref] [PubMed]
- Chabes AL, Björklund S, Thelander L S. Phase-specific transcription of the mouse ribonucleotide reductase R2 gene requires both a proximal repressive E2F-binding site and an upstream promoter activating region. J Biol Chem 2004;279:10796-807. [Crossref] [PubMed]
- Fu YP, Kohaar I, Moore LE, et al. The 19q12 bladder cancer GWAS signal: association with cyclin E function and aggressive disease. Cancer Res 2014;74:5808-18. [Crossref] [PubMed]
- Fang Z, Gong C, Liu H, et al. E2F1 promote the aggressiveness of human colorectal cancer by activating the ribonucleotide reductase small subunit M2. Biochem Biophys Res Commun 2015;464:407-15. [Crossref] [PubMed]
- Xia G, Wang H, Song Z, et al. Gambogic acid sensitizes gemcitabine efficacy in pancreatic cancer by reducing the expression of ribonucleotide reductase subunit-M2 (RRM2). J Exp Clin Cancer Res 2017;36:107. [Crossref] [PubMed]
- Kawatsuki A, Yasunaga JI, Mitobe Y, et al. HTLV-1 bZIP factor protein targets the Rb/E2F-1 pathway to promote proliferation and apoptosis of primary CD4(+) T cells. Oncogene 2016;35:4509-17. [Crossref] [PubMed]
- Zhao S, Shen W, Yu J, et al. TBX21 predicts prognosis of patients and drives cancer stem cell maintenance via the TBX21-IL-4 pathway in lung adenocarcinoma. Stem Cell Res Ther 2018;9:89. [Crossref] [PubMed]
- Sui Q, Liu D, Jiang W, et al. Dickkopf 1 impairs the tumor response to PD-1 blockade by inactivating CD8+ T cells in deficient mismatch repair colorectal cancer. J Immunother Cancer 2021;9:e001498. [Crossref] [PubMed]
- Liu P, Zhang X, Li Z, et al. A significant role of transcription factors E2F in inflammation and tumorigenesis of nasopharyngeal carcinoma. Biochem Biophys Res Commun 2020;524:816-24. [Crossref] [PubMed]
- Fang F, Wang Y, Li R, et al. Transcription factor E2F1 suppresses dendritic cell maturation. J Immunol 2010;184:6084-91. [Crossref] [PubMed]
- Gong D, Feng PC, Ke XF, et al. Silencing Long Non-coding RNA LINC01224 Inhibits Hepatocellular Carcinoma Progression via MicroRNA-330-5p-Induced Inhibition of CHEK1. Mol Ther Nucleic Acids 2020;19:482-97. [Crossref] [PubMed]
- Luo T, Gao Y, Zhangyuan G, et al. lncRNA PCBP1-AS1 Aggravates the Progression of Hepatocellular Carcinoma via Regulating PCBP1/PRL-3/AKT Pathway. Cancer Manag Res 2020;12:5395-408. [Crossref] [PubMed]
- Yang X, Sun L, Wang L, et al. LncRNA SNHG7 accelerates the proliferation, migration and invasion of hepatocellular carcinoma cells via regulating miR-122-5p and RPL4. Biomed Pharmacother 2019;118:109386. [Crossref] [PubMed]
- Zhou Y, Li K, Dai T, et al. Long non-coding RNA HCP5 functions as a sponge of miR-29b-3p and promotes cell growth and metastasis in hepatocellular carcinoma through upregulating DNMT3A. Aging (Albany NY) 2021;13:16267-86. [Crossref] [PubMed]
- Wu H, Zhang W, Wu Z, et al. miR-29c-3p regulates DNMT3B and LATS1 methylation to inhibit tumor progression in hepatocellular carcinoma. Cell Death Dis 2019;10:48. [Crossref] [PubMed]
- Lv T, Jiang L, Kong L, et al. MicroRNA-29c-3p acts as a tumor suppressor gene and inhibits tumor progression in hepatocellular carcinoma by targeting TRIM31. Oncol Rep 2020;43:953-64. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)