Is early response by 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography a predictor of long-term outcome in patients with metastatic colorectal cancer?
Introduction
Colorectal cancer (CRC) is the third most common cancer in developed countries and is the second commonest cause of cancer deaths. CRC is highly treatable and often curable when localized to the bowel, however in metastatic or recurrent CRC the treatment is palliative (1).
The treatment of metastatic CRC is based on chemotherapy with fluoropyrimidine and oxaliplatin or irinotecan (2-5), associated with monoclonal antibodies: bevacizumab, cetuximab, panitumumab or the antiangiogenic agent aflibercept (6-11). Systemic treatment in metastatic CRC is not a curative approach, except when complete resection of metastases is feasible, leading to longer survival (12). Identifying responder patients to chemotherapy regimen is a critical goal in solid tumors, once this approach can save non-responders from long and toxic treatments, optimizing treatment selection.
The standard response assessment method in Medical Oncology is Response Evaluation Criteria in Solid Tumors (RECIST) (13,14), using tumor measurement by conventional imaging: computed tomography or magnetic resonance imaging (MRI). However, the accuracy of RECIST has been questioned with the use of biological agents, whereas they are more cytostatic than cytotoxic drugs, there can be tumor shrinkage without size changing due to post-therapy fibrosis (15). Furthermore, it can take long period to observe response in anatomic imaging, usually performed after eight weeks of treatment. The 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography (18FDG-PET-CT), as a technique to evaluate metastatic lesions in a metabolic scenario, appears attractive to assess early response. Currently 18FDG-PET-CT has been used in metastatic CRC for staging before surgery, restaging and recurrence localization in patients with high serum carcinoembryonic antigen levels (CEA) and normal conventional imaging (16,17).
18FDG-PET-CT is an imaging that quantifies the metabolic process in cancer cells; hence, it indirectly measures cellular proliferation and the effectiveness of the treatment (18,19).
18FDG-PET-CT has established role in the early response evaluation in lymphoma, breast cancer, localized rectal cancer and operable metastatic CRC (20-25). PET-CT findings can induce treatment changes in metastatic CRC patients, but it lacks robust trials in the current context of new therapies with biological agents (26,27). de Geus-Oei et al. investigated response with FDG-PET-CT after two months of chemotherapy in metastatic colorectal patients. It was observed increasing in progression and death rates in the patients with worse metabolic response (28). They had previously demonstrated that in colorectal liver metastases a low FDG uptake prior the treatment predict a benefit survival (29).
Tam et al. had also observed that in liver colorectal metastases patients, a high standardized uptake value (SUV) prior therapy correlated to a shorter progression free survival (PFS), independently of other prognostic factors (30). However this analysis was retrospective and the PET-CT was not used for measure tumor response after chemotherapy.
Byström et al. studied PET-CT response after two cycles of chemotherapy without monoclonal antibody. There was no correlation between metabolic response and time to progression or overall survival (OS) (31).
Hendlisz et al. analyzed the impact of PET-CT after one cycle of chemotherapy [5-fluorouracil, leucovorin (folinic acid), irinotecan (FOLFIRI), 5-fluorouracil, leucovorin (folinic acid), oxaliplatin (FOLFOX) or capecitabine] in metastatic CRC and showed relation between metabolic response and OS. Nevertheless only 16% of these patients had used biological drugs associated to chemotherapy (32).
Recently, Lastoria et al. evaluated thirty-one patients with liver metastases from CRC, receiving pre-operative treatment with FOLFIRI and bevacizumab. They found association between metabolic responses based on FDG-PET-CT after one cycle and PFS and OS (33).
In sum, the previous trials had included heterogeneous chemotherapeutic regimens without biological agents in the majority of the patients and used different metabolic response criteria, except the latest study by Lastoria et al. However, their population is a select group with resectable liver metastases treated with a unique schema of chemotherapy (Table 1).

Full table
The aim of this present study was to evaluate the prognostic value of early response by FDG-PET-CT in metastatic CRC patients on first-line chemotherapy with monoclonal antibodies.
Methods
Patients
This study included patients with metastatic CRC, aged 18–75 years old, with Eastern Cooperative Group performance status of 0 or 1, scheduled to receive first-line treatment with chemotherapy associated with a monoclonal antibody at AC Camargo Cancer Center, in the Medical Oncology Department, from March 2009 to October 2011.
The Institutional Review Board of AC Camargo Cancer Center approved the study and written informed consent was obtained from all patients.
All patients had histopathological diagnosis of colorectal adenocarcinoma, including the KRAS status analysis (codons 12 and 13) and at least one metastatic lesion on computed tomography or MRI.
Treatment
The Chemotherapy schema were based on physician’s choice and administered intravenously every 14 days: FOLFIRI-irinotecan (180 mg/m2 intravenous infusion on day 1), leucovorin (200 mg/m2 intravenous infusion on day 1), 5-fluorouracil (400 mg/m2 by intravenous bolus on day 1), and 5-fluorouracil (2,400 mg/m2 46-h continuous infusion) or FOLFOX-oxaliplatin (85 mg/m2 intravenous infusion on day 1), leucovorin (200 mg/m2 intravenous infusion on day 1), 5-fluorouracil (400 mg/m2 by intravenous bolus on day 1), and 5-fluorouracil (2,400 mg/m2 46-h continuous infusion); bevacizumab was administered at 5 mg/kg by intravenous infusion over 90 min, at the first cycle, then, if tolerated, over 60 min or cetuximab was administered at 400 mg/m2 as initial dose and 250 mg/m2 weekly or every two weeks at 500 mg/m2 as intravenous infusion over 120 min.
Imaging evaluation with CT or MRI was performed according to physician’s judgment and analyzed by expert radiologists using RECIST criteria.
18F-2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography (18FDG-PET-CT)
The patients underwent the first 18FDG-PET-CT before starting the treatment in addition to conventional images (CT or MRI) of chest, abdomen and pelvis, besides routine laboratory tests. A second PET-CT was performed immediately before the third cycle of therapy, from twenty-five to thirty days after the beginning of treatment.
The images were obtained on a Gemini PET/CT (Philips Medical Systems) with whole-body PET scanner. The patients fasted for at least 6 hours prior PET imaging. Serum glucose level was measured and it had to be lower than 200 mg/dL for all patients. After that, the patients received an intravenous injection of 5.0 megabecquerels per kilogram (MBq/Kg) of 18F-FDG, and the first images were acquired approximately 90 min after the radiotracer injection. The patients were laid in supine position during the study, and were comfortably positioned on the scanner table with both arms at their side.
The regions of interest (ROIs) were manually drawn on the slice with the highest radioactivity concentration. The lesions were analyzed using the maximum SUVmax method, which was defined as the maximum tissue concentration of FDG (fluor-deoxyglucose) in the ROI. The SUV max was calculated by the formula: tissue concentration (MBq/g)/injected dose (MBq)/body weight (g).
An experienced nuclear medicine physician interpreted the whole-body PET images, and was blinded for patient’s history, clinical findings, and conventional imaging.
It was considered the difference the two 18FDG-PET-CT for the early response assessment (delta ∆ SUV). In patients with multiple metastases, it was chosen randomly three to five lesions with 18FDG-PET-CT uptake. Patients with less than five lesions: all the lesions were evaluated. European Organization for Research and Treatment in Cancer (EORTC) criteria for PET-CT were used: partial response is when delta SUV drop more than 25%, disease progression is when delta SUV increases more than 25% or appearance of new metastatic lesions and stable disease when the SUV decrease be less than 25% or the increase be less than 25% (34).
A responder patient was defined as someone that had partial or complete response.
Statistical analysis
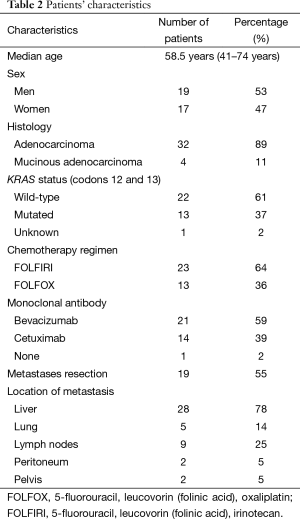
Demographic and clinical characteristics were summarized by medians and frequencies (Table 2). PFS was defined as the interval between diagnosis of metastasis and first documentation of progression or death. OS was calculated from diagnosis of metastasis to date of death due to any reason. Patients alive were censored at the last time point.
Full table
Differences in OS and PFS were estimated using the Kaplan-Meier Method and were compared between the groups by log-rank test. SPSS for Windows version 22.0 was used for data analysis (SPSS Inc., Chicago, IL, USA).
Pathological and clinical variables (age, sex, histologic subtypes, KRAS status, chemotherapy regimen, monoclonal antibody, resection of metastases and response by RECIST) were correlated with changes in SUV, as well as OS and PFS. Fisher’s method was used to correlate response by 18FDG-PET-CT and demographics parameters. The level of significance was set at 0.05.
Results
Patients’ characteristics
Thirty-six patients with metastatic CRC were included in this prospective trial, with a median age of 58.5 years (range, 41–74 years). Demographic characteristics and treatment regimens are showed in Table 2. Five patients started monoclonal antibody only in the second cycle of chemotherapy, one patient started after the second cycle and another patient did not use monoclonal antibodies, the last two patients were excluded from analysis. Statistical analysis was performed with thirty-four patients that had used monoclonal antibody since the first or second cycle of chemotherapy. Complete metastases resection was controlled as covariate for the measurement of delta SUV cut-off value (35).
18F-2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography (18FDG-PET-CT)
Based on EORTC criteria for 18FDG-PET-CT response evaluation, there were 82% responder patients and 18% non-responder patients. With median follow-up of 24 months, there is no difference in PFS (15.5 vs. 13.3 months, P=0.42) neither OS (55.7 months and not reached for non-responders, P=0.42) between responders and non-responders (Figure 1).
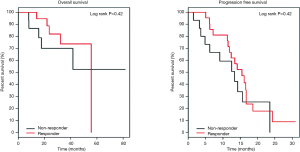
None of the variables (KRAS status, histology subtype, early response by 18FDG-PET-CT and chemotherapy regimen) was predictor of tumor progression in this series. Late response evaluation by conventional imaging (CT or MRI) was predictive of worse PFS (RR =8.12, P<0.01) and worse OS (RR =10.81, P<0.01) for non-responder patients, and metastases resection was a good prognostic factor.
Considering different thresholds for SUV response in the previous trials that evaluated metabolic response, for this population, it was identified a predictive SUV cut-off value by the method of Martingale-Residual (35). It was found a cut-off value of 55% for OS and 60% for PFS. Even thus, the curves of OS and PFS did not show difference between responder and non-responder patients.
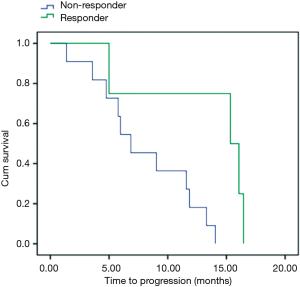
As the majority of our patients (55%) were submitted to liver metastases resection, and this has a remarkable effect in prognosis, it was done an unplanned analysis of patient subgroup that had not had surgery. In fifteen patients, using the cut-off delta SUV <−60% for PFS, we had four responder patients and eleven non-responders (Figure 2). PFS for responder was 15.3 vs. 6.8 months for non-responders patients (P=0.02). OS was 21 months for responder and 15 months for non-responder patients (P=0.86).
Discussion
Apart from KRAS and NRAS mutation status, that selects potential responder patient to anti-epidermic growth factor receptor (EGFR) therapy, there are no more biomarkers available in daily practice for metastatic CRC. Therefore, early tumor shrinkage has been studied as a marker of response in metastatic CRC. It is a challenge selecting non-responder patients to change therapy prematurely, avoiding toxicity and excessive cost without negatively affecting PFS and OS (36). A questionable point is: which is the most suitable method to response evaluation with biological drugs? Antiangiogenic agents cause interstitial remodeling and tumor activity decreasing without change in tumor diameters. 18FDG-PET-CT, as a metabolic imaging, seems extremely attractive to predict effectiveness of treatment in metastatic CRC.
In Non-Hodgkin lymphoma, PET-CT is standard as response predict after one or two cycles of chemotherapy. In some protocols, patients that have negative PET after two cycles of chemotherapy can be treated with less intensive regimen (37).
In CRC, Piessevaux et al. reported the impact of tumor decreasing more than 20% in PFS and OS in patients with wild-type KRAS mCRC treated with cetuximab plus FOLFIRI or FOLFOX-4. Different from our study, this analysis was done with conventional imaging at eight weeks from beginning of treatment (38). Choi et al. showed in advanced CRC patients that absence of FDG uptake on follow-up PET scans was associated with markedly longer OS and time to progression in this population (39).
Early PET-CT response is still investigational in metastatic CRC. Questions to be answered are the standardization of PET-CT response criteria, time to perform PET-CT, number of lesions to be evaluated and how to interpret the response of different biological agents (antiangiogenic and anti-EGFR drugs).
We evaluated early metabolic response by 18FDG-PET-CT as a predictor of long-term outcome in metastatic CRC patients treated in first line with chemotherapy plus monoclonal antibodies and we did not find positive correlation, following the negative results after two cycles of chemotherapy (without monoclonal antibodies) reported by Byström et al. (31). Some hypothesis can explain these findings: metabolic response after two cycles can be transient and right after the tumor develops resistance and long-term clinical results can not be predictable; besides that, SUV threshold and PET-CT timing are not standard through the prospective studies, and the majority of patients have partial response or stable disease with first line therapy, therefore PET-CT may not make difference in the first line response assessment. Other confounding factor is the heterogeneity of metabolic response within body tumor load. In a recent trial, in metastatic CRC patients treated with capecitabine and sorafenib, a PET-CT performed after the first cycle of therapy with at least one metabolically refractory lesion is associated with poor outcome (40).
An interesting result in the present study is the unplanned analysis of the patients had not submitted to metastases resection. In this group, early responder patients had longer PFS comparing to non-responder patients (15.3 vs. 6.8 months, P=0.02). For this analysis it was considered the ∆ SUV of −60%, notwithstanding there were few patients in this trial. This threshold is higher than EORTC criteria and up to now the most appropriate cut-off values and standardization for PET-CT is under investigation. This point is pertinent and need to be confirmed by large prospective trials. Considering metastases resection a common practice in metastatic CRC, future studies should investigate the real value of PET-CT in non-operable patients and use PFS as primary endpoint, and the positive impact of PET-CT in this population need to be confirmed.
Conclusions
According to our findings, early metabolic response by 18FDG-PET-CT is not a predictor of long-term outcome for patients with metastatic CRC treated in the first-line chemotherapy with a monoclonal antibody. Our results are, of course, limited by small sample size. For patients with no-resectable metastases, we found a better PFS for responders. However, we cannot draw conclusions for this subgroup, once this analysis was not planned.
Acknowledgements
We thank to all the patients involved in this trial and all the physicians from AC Camargo Cancer Center who taken part in this project. We are also grateful to the Brazilian Institution for Nuclear Energy, IPEN (Instituto de Pesquisa Energéticas e Nucleares), for donate the F-18-FDG (2-fluoro-2-deoxi-D-glucose) to perform the PET-CT, making this trial possible.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society: Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society, 2015. Available online: , acessed on October 30, 2015.http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 1997;15:808-15. [PubMed]
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905-14. [Crossref] [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [Crossref] [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [Crossref] [PubMed]
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466-75. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [Crossref] [PubMed]
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [Crossref] [PubMed]
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22, discussion 722-4. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Burton A. REGIST: right time to renovate? Eur J Cancer 2007;43:1642. [PubMed]
- Lin M, Wong K, Ng WL, et al. Positron emission tomography and colorectal cancer. Crit Rev Oncol Hematol 2011;77:30-47. [Crossref] [PubMed]
- Kong G, Jackson C, Koh DM, et al. The use of 18F-FDG PET/CT in colorectal liver metastases--comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging 2008;35:1323-9. [Crossref] [PubMed]
- Rigo P, Paulus P, Kaschten BJ, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med 1996;23:1641-74. [Crossref] [PubMed]
- Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med 2006;354:496-507. [Crossref] [PubMed]
- Mikhaeel NG, Hutchings M, Fields PA, et al. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol 2005;16:1514-23. [Crossref] [PubMed]
- Dunnwald LK, Gralow JR, Ellis GK, et al. Tumor metabolism and blood flow changes by positron emission tomography: relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol 2008;26:4449-57. [Crossref] [PubMed]
- Cascini GL, Avallone A, Delrio P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med 2006;47:1241-8. [PubMed]
- Melton GB, Lavely WC, Jacene HA, et al. Efficacy of preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography for assessing primary rectal cancer response to neoadjuvant therapy. J Gastrointest Surg 2007;11:961-9; discussion 969. [Crossref] [PubMed]
- Briggs RH, Chowdhury FU, Lodge JP, et al. Clinical impact of FDG PET-CT in patients with potentially operable metastatic colorectal cancer. Clin Radiol 2011;66:1167-74. [Crossref] [PubMed]
- Lau LF, Williams DS, Lee ST, et al. Metabolic response to preoperative chemotherapy predicts prognosis for patients undergoing surgical resection of colorectal cancer metastatic to the liver. Ann Surg Oncol 2014;21:2420-8. [Crossref] [PubMed]
- de Geus-Oei LF, Vriens D, van Laarhoven HW, et al. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med 2009;50 Suppl 1:43S-54S. [Crossref] [PubMed]
- Artiko V, Odalovic S, Sobic-Saranovic D, et al. Can (18)F-FDG PET/CT scan change treatment planning and be prognostic in recurrent colorectal carcinoma? A prospective and follow-up study. Hell J Nucl Med 2015;18:35-41. [PubMed]
- de Geus-Oei LF, van Laarhoven HW, Visser EP, et al. Chemotherapy response evaluation with FDG-PET in patients with colorectal cancer. Ann Oncol 2008;19:348-52. [Crossref] [PubMed]
- de Geus-Oei LF, Wiering B, Krabbe PF, et al. FDG-PET for prediction of survival of patients with metastatic colorectal carcinoma. Ann Oncol 2006;17:1650-5. [Crossref] [PubMed]
- Tam HH, Cook GJ, Chau I, et al. The role of routine clinical pretreatment 18F-FDG PET/CT in predicting outcome of colorectal liver metastasis. Clin Nucl Med 2015;40:e259-64. [Crossref] [PubMed]
- Byström P, Berglund A, Garske U, et al. Early prediction of response to first-line chemotherapy by sequential [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with advanced colorectal cancer. Ann Oncol 2009;20:1057-61. [Crossref] [PubMed]
- Hendlisz A, Golfinopoulos V, Garcia C, et al. Serial FDG-PET/CT for early outcome prediction in patients with metastatic colorectal cancer undergoing chemotherapy. Ann Oncol 2012;23:1687-93. [Crossref] [PubMed]
- Lastoria S, Piccirillo MC, Caracò C, et al. Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med 2013;54:2062-9. [Crossref] [PubMed]
- Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773-82. [Crossref] [PubMed]
- Klein JP, Wu JT. Discretizing a continuous covariate in survival studies. In: Balakrishnan N, Rao CR, editors. Handbook of Statistics 23: Advances in Survival Analysis. New York: Elsevier, 2004:27-42.
- Suzuki C, Blomqvist L, Sundin A, et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol 2012;23:948-54. [Crossref] [PubMed]
- Haioun C, Itti E, Rahmouni A, Brice P, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 2005;106:1376-81. [Crossref] [PubMed]
- Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2013;31:3764-75. [Crossref] [PubMed]
- Choi M, Kollepara SL, Heilbrun LK, et al. PET scans as a predictive marker of survival in advanced colorectal cancer. Clin Colorectal Cancer 2015;14:35-40. [Crossref] [PubMed]
- Hendlisz A, Deleporte A, Delaunoit T, et al. The Prognostic Significance of Metabolic Response Heterogeneity in Metastatic Colorectal Cancer. PLoS One 2015;10:e0138341. [Crossref] [PubMed]


