Is distance to chemotherapy an obstacle to adjuvant care among the N.C. Medicaid—enrolled colon cancer patients?
Introduction
Colorectal cancer is the third most common cancer in the United States and the third leading cause for cancer mortality for both men and women (1). Its early detection and treatment, including adjuvant chemotherapy, may improve survival (2,3). However, patients with low socioeconomic status (SES) and those enrolled in Medicaid have worse access to colon cancer screening, worse access to treatment and lower survival rates than higher SES groups (4-7). While adjuvant chemotherapy is recommended for treatment of all stage III (regional) colon cancer patients and high-risk cases of SEER stage II (local) colon cancer, many Medicaid-enrolled patients do not receive the recommended chemotherapy treatment (5,8). In Etzioni et al. 2008’s systematic literature review, studies found chemotherapy receipt for stage III colon cancer patients to be linked to age, gender, race, SES, insurance status, rural vs. urban community, and the academic center status and size of the medical care facility (9). Two of the included studies addressed geographic variation, but none examined the distance to provider as a potential obstacle to receiving chemotherapy (9-11). In our study we address whether the distance from a patient’s residence to the nearest chemotherapy provider is associated with the likelihood of chemotherapy receipt for low-income, Medicaid-enrolled patients diagnosed with stage III and stage II cancer.
The distance to provider is a proxy for costs associated with traveling for chemotherapy treatments. Patients who live further away from the provider spend more time travelling, may incur higher travel expenses, and face more inconveniences that lower their likelihood of receiving chemotherapy. Several studies addressed the relationship between travel burden and colon cancer care, as well as utilization of medical care for other types of cancer and medical conditions. Aas found that colorectal cancer screening participation in Norway decreased when travel expenses were high (12). Winget et al. observed a geographic variation in colon cancer patients’ consultations with oncologists in Canada but could not explain it by variation in the distance to the nearest cancer facility (13). Onega et al. found that rural dwellers and those residing in the Southern region of the US travelled longer distances to colon cancer care providers but stated that the relationship between travel burden, care and outcomes for colon cancer patients is not clearly understood (14). In a UK based study by Jones et al. rectal and lung cancer patients were less likely to receive chemotherapy if the lived in the longest hospital travel time quartile, but the result for colon cancer was just short of being statistically significant (15). Longer distance to providers was associated with a later diagnosis stage for breast cancer and lower likelihood of breast cancer screening and breast-conserving therapy in the US and UK (15-19). US patients with myocardial infarction who lived within a shorter distance to the nearest specialized hospital relative to any nearest hospital were more likely to receive the appropriate catheterization and revascularization treatment (20-21).
Our topic is especially relevant for Medicaid patients. While Medicaid covers direct medical costs, patients’ travel burden may be significant due to low income and limited means of transportation. According to Freeman et al., 31% of children enrolled in Medicaid Managed Care living in rural areas and 21% of those living in urban areas had an unmet medical care need because the care was too far away or they had no transportation (22). Scoggins et al. found that time-to-treatment of Medicaid colorectal cancer patients in Washington State was associated with travel burden (16). Bradley et al. found that low-income Medicaid patients were less likely to initiate and complete chemotherapy than Medicare patients and posit that the lack of transportation support services to help them get to physician offices is one of the plausible reasons why (5).
Our study contributes to the literature on access to care for low income colon cancer patients by addressing an under-explored potential determinant of adjuvant chemotherapy treatment, the distance to the nearest chemotherapy provider. Using merged multiple data sources we were able to obtain both patient and provider residential addresses and create a unique distance-to-nearest-provider value for each patient. Such an approach is not common in the existing literature where the norm is to use the geographic center of a zip code as the identical address for all patients residing in the zip code. Further, this study was conducted using a relatively homogenous sample with respect to income and insurance status. In order to qualify for the means-tested Medicaid all patients must have been low income individuals, and because they all resided in North Carolina, their Medicaid care was subject to the same legislative policies and rules. Such sample homogeneity allowed us to more precisely evaluate whether the distance to provider is a determinant of access to chemotherapy.
Methods
Conceptual framework
Chemotherapy is expected to improve survival, but it also imposes costs on the patient, which has a negative effect on one’s wellbeing. The distance to provider is a proxy for non-medical and time costs of transportation. Patients who live further away from the provider spend more time travelling, may incur higher travel expenses, and face more inconveniences. We hypothesize that the larger the distance to the nearest chemotherapy provider, the larger the travel and time costs of chemotherapy and the lower the likelihood of receiving chemotherapy.
Study design and data sources
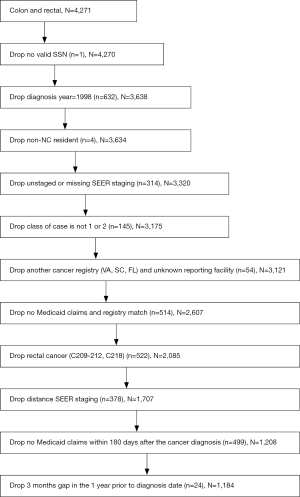
The dataset was created from four data sources: the North Carolina Central Cancer Registry, North Carolina Medicaid/Medicare Claims, American Hospital Directory and Census 2000. All NC residents diagnosed with cancer are registered in the NC Central Cancer Registry by law, and their demographic characteristics and first courses of treatment are recorded in this file. The registry file was merged with the NC Medicaid eligibility file by patient’s social security number, which was de-identified prior to our analysis. The Medicare and Medicaid claims files were cross-linked, such that individuals ages 65 and older dually-enrolled in Medicaid and Medicare have complete claims data. The NC Medicaid file was merged with the American Hospital Directory file by the facility identification number. We used the ESRI ArcGIS software to geocode patient addresses in the cancer registry and merged it with Census 2000 summary file 3 to obtain the urban/rural status and community poverty rank. The research was approved by the Wake Forest University Health Sciences and Davidson College Institutional Review Boards, the NC Division of Medicare and Medicaid, and the North Carolina Central Cancer Registry.
The final sample consists of 1,184 individuals, both men and women. Following Foley et al., the individuals were diagnosed with first primary, SEER-staged (C180, C181, C182, C183, C184, C185, C186, C187, C188, C189, C199) local or regional colon cancer in the years 1999-2002 and resided in North Carolina (8). They were Medicaid recipients for the whole duration of the study and filed a Medicaid claim within 180 days following their cancer diagnosis. All patients over the age of 65 also have Medicare claims that crossed-over to the Medicaid claims files (supplementary materials).
The outcome variable is the receipt of chemotherapy within 365 days of diagnosis. A patient received chemotherapy if at least one of the following Healthcare Common Procedure Coding Systems procedure codes or ICD-9 codes appeared in a claim: Jxxx, 964xx, or 965xx series; G0345 to G0363; Q0083 to Q0085; RC331 to RC335; S9329; and W8222, and ICD-9 codes 99.25, V58.1x, V66.2, and V67.2. The NCCN-v1.2011 Treatment Guidelines recommend adjuvant chemotherapy for patients with SEER-staged regional cancer and consideration of chemotherapy for selected high risk patients with SEER-staged local cancer. For example, a local cancer chemotherapy referral may be justified by family history or participation in a clinical trial. Because of different guidelines, we analyzed regional and local cancer patients also as two separate samples.
The main explanatory variable of interest is the distance from the patient’s residential address to the nearest chemotherapy provider, measured in brackets of (I) 0 to <2 miles (reference group); (II) ≥2 to <5 miles; (III) ≥5 to <15 miles; and (IV) ≥15 miles. In an alternative specification, two more brackets were added: ≥15 to <20 miles, and ≥20 miles. We took the following steps to create the distance variable. We obtained patient residential addresses from the NC Cancer Registry file. We identified providers with Medicaid chemotherapy claims in the Medicaid/Medicare Claims detail set and obtained their addresses from the Medicaid provider file. All addresses were geocoded using ArcGIS 9.3 Desktop ArcMap, and distances were computed using SAS 9.1.3 as the straight line distance between the longitude and latitude coordinates of the patients’ and providers’ addresses. We then assigned the nearest chemotherapy provider to each patient. To account for opening and closing of chemotherapy facilities, each patient was assigned the nearest distance chemotherapy provider out of a pool of facilities that filed at least one chemotherapy claim for Medicaid patients during the year of the patient’s diagnosis or the following year. For example, out of 538 chemotherapy facilities in NC with claims made during 1999–2003, 223 facilities submitted a claim in 1999–2000. Thus patients diagnosed in 1999 were assigned the nearest provider out of this pool of 223 facilities. In 2000–2001, 272 chemotherapy facilities submitted a claim; in 2001–2002, 297; and in 2002–2003, 288.
A total of 140 addresses could not be geocoded in the NC Cancer Registry file (e.g., P.O. Box addresses) and thus 140 individuals in the sample have missing values for the distance to provider. These observations could be excluded from the analysis only if geocoded addresses were missing randomly; otherwise their exclusion would result in biased estimates. We performed the statistical analysis using a sample that included the observations with missing values, in which we replaced the missing distance variable values with a 0 and created a missing variable dichotomous indicator set to a 1 for observations with the missing values.
Control variables
Control variables including patient, provider and community characteristics. The NC Cancer Registry was the source for patient’s age at diagnosis (<65 years, 65–75 years and ≥75), gender (male, female) and race (white, other race). The Medicaid/Medicare Claims detail file was the source for the non-cancer Charlson Comorbidity Index Score (dichotomous variable representing score =0 and score =1+) constructed from patients’ pre-cancer comorbid conditions using ICD-9 codes 1 year prior to the diagnosis (23). It serves as a proxy for pre-existing health conditions that constitute a higher mortality risk unrelated to cancer.
Provider characteristics include the reporting hospital’s total surgery volume and its membership in the University Health Consortium, recorded in the American Hospital Directory. These characteristics are proxies for chemotherapy referral patterns of physicians who initially diagnosed and/or surgically treated colon cancer patients in this sample. Most colon cancer patients get the stage diagnosis and undergo cancer surgery at a hospital that reports their diagnosis to the North Carolina Central Cancer registry. Then they are often referred by their reporting hospital physicians for medical oncology consultation and adjuvant chemotherapy that takes place elsewhere at a specialized chemotherapy facility. The general assumption is that physicians in high surgery volume hospitals and university-affiliated hospitals might be more exposed to guidance-consistent care and referrals.
The community characteristics include the poverty level of the patient’s Census block group from the US Census file, assigned to the patient’s geocoded address using geographic identifiers. If a block level match was not made, 5-digit and 3-digit zip code level poverty data was used (12% match). The urban vs. rural dichotomous variable was created by mapping the geocoded patient’s addresses with the USA Urban Areas layer available in ArcGIS that presents the Census 2000 Urbanized Area and Urban Clusters.
Statistical analysis
Statistical analysis includes descriptive statistics of patient, provider and community characteristics and logistic regression models estimating the receipt of chemotherapy on the distance to nearest provider variables and control variables. Statistical significance was indicated by a two-sided alpha level of 0.05. All data was analyzed using Stata 11. Odds ratios (ORs) and 95% confidence intervals (CI) are reported.
Results
Descriptive characteristics
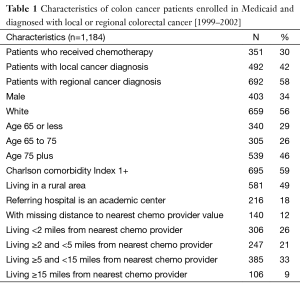
The sample consisted of 1,184 individuals, out of which 30% received chemotherapy (Table 1). About 34% were male, 56% were white, and 59% lived in a rural area. About 42% of patients were diagnosed with the local stage and 58% with the regional stage of colon cancer. The 12% of local cancer patients and 43% of regional cancer patients received chemotherapy (Table 2). Among local cancer patients who received chemotherapy, 48% lived within 2 miles from the nearest provider while only 2% lived at least 15 miles away. In contrast, among local cancer patients who did not receive chemotherapy, only 35% lived within 2 miles from the nearest provider and 11% lived ≥15 miles away. Among regional cancer patients, the proportion of patients who lived close vs. far from chemotherapy providers is similar for the groups who did and did not receive chemotherapy. Interestingly, while the average distance from the patient’s residence to the nearest chemotherapy provider accepting Medicaid patients was only 6.4 miles, patients who actually received chemotherapy traveled on average almost 15 miles to their provider.
Full table

Full table
Logistic regression: distance to the nearest provider and receipt of chemotherapy
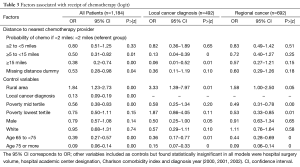
Compared to the referent group of patients living within 2 miles from the nearest chemotherapy, the odds of receiving chemotherapy fell as the distance to the nearest chemotherapy provider increased (Table 3). The OR of receiving chemotherapy for those living ≥5 to <15 away was 0.50 (95% CI, 0.31–0.82), and the OR for those living ≥15 miles away was 0.38 (95% CI, 0.20–0.74). Missing geocoded address was associated with lower odds of chemotherapy receipt (OR =0.53, 95% CI, 0.28–0.98).
Full table
Patients were also less likely to receive chemotherapy if they were diagnosed with the local stage of colon cancer (OR =0.13, 95% CI, 0.09–0.19), if they were older (OR =0.39, 95% CI, 0.27–0.57 for those 65 to <75 years old, and OR =0.09, 95% CI, 0.06–0.14 for those 75 years and older) and if they lived in a community ranked in the mid poverty tertile (OR =0.56, 95% CI, 0.38–0.83). Those living in rural communities were more likely to receive chemotherapy (OR =1.84, 95%CI, 1.23–2.73) than patients in urban areas. We found no statistically significant association between chemotherapy receipt and the reporting hospital’s attributes, patient’s gender, race and Charlson comorbidity index.
Distance to provider and diagnosis stage
The NCCN-v1.2011 and v2.2014 Treatment Guidelines recommend adjuvant chemotherapy treatment for patients with SEER-staged III regional cancer and consideration of chemotherapy for patients with high-risk features of SEER-staged II local cancer. While chemotherapy is recommended for all patients diagnosed at regional cancer stage, it is not uniformly recommended for patients diagnosed at local stage. Consequently, physician referral patterns and patient incentives to pursue chemotherapy treatments may differ across these two groups. We thus stratified the sample into the local cancer and regional cancer groups and evaluated the distance-chemotherapy association separately.
We found that distance to the nearest chemotherapy provider was not associated with lower chemotherapy receipt for regional cancer patients (Table 3). On the other hand, it was a statistically significant determinant of chemotherapy, with a large magnitude, for patients with local cancer diagnosis (Table 3). The odds of receiving chemotherapy fell with distance for patients with local cancer: OR for those living ≥5 to <15 miles away was 0.13 (95% CI, 0.04–0.39), and OR for those living ≥15 miles away was 0.06 (95% CI, 0.01–0.52). For both the local cancer and regional cancer groups, old age continued to be a statistically significant determinant of lower likelihood of chemotherapy receipt, while living in a rural area was associated with a higher likelihood of receiving chemotherapy.
Distance to provider in rural and urban areas
We next distinguished between the distance-chemotherapy associations in rural vs. urban areas to acknowledge potentially different transportation challenges facing patients. Chemotherapy receipt of urban dwellers with regional cancer was not associated with distance to the nearest provider (Table 4). For regional cancer patients in rural areas, a statistically significant negative distance-chemotherapy relationship emerged only for patients living furthest away. The OR for those living ≥20 miles from the nearest chemotherapy provider was 0.08 (95% CI, 0.01–0.72). The missing distance indicator was statistically significant for the sample of patients living in rural areas, implying that rural residents with missing geocoded (residential) addresses, who most likely had PO boxes, were less likely to receive chemotherapy. This result confirms that geocoded addresses were not missing randomly in the sample, and that the inclusion of the missing distance indicator in the model was justified.

Full table
For local cancer patients, we continued to find a strong negative association between the distance to the nearest provider and chemotherapy receipt both in rural and urban areas (Table 5). In urban areas, none of the local cancer patients living more than 5 miles away from the nearest provider received chemotherapy. In rural areas, the OR of receiving chemotherapy for those living ≥5 to <15 miles away was 0.05 (95% CI, 0.01–0.35), and the OR was 0.03 for those living ≥15 miles from the nearest provider (95% CI, 0.00–0.49).

Full table
Discussion
We found that the distance to the nearest provider helps explain differences in access to chemotherapy for some groups of Medicaid-enrolled colon cancer patients. First, local cancer patients in rural areas were less likely to receive chemotherapy as distance to the nearest chemotherapy provider increased. Second, none of local cancer patients living in urban areas more than 5 miles from the nearest chemotherapy provider received chemotherapy. Third, regional cancer patients living in rural areas were less likely to receive chemotherapy only if they lived more than 20 miles away from the nearest chemotherapy provider. Last but not least, we found no evidence of association between distance to the nearest provider and chemotherapy receipt for regional cancer patients in urban areas and those living in rural areas within 20 miles from the nearest chemotherapy provider.
The stronger negative distance-chemotherapy association found for local cancer patients than regional cancer patients may reflect that, consistent with recommended treatment guidelines, chemotherapy is perceived by physicians and patients to have large benefit for regional cancer, while chemotherapy is an optional treatment with a lower potential benefit relative to transportation costs for treating local cancer. Higher likelihood of chemotherapy utilization by local cancer patients living close to providers may reflect unobserved health and participation in clinical trials, participants of which are likely to live within an easy reach of treatment facilities, but it may also be a sign of overuse. More research is needed to better understand reasons for referring local cancer patients for chemotherapy, addressing variation in physician practicing styles and chemotherapy provider concentration, as well as the effect of chemotherapy on local cancer patients’ survival.
Surprisingly, we found that both regional and local cancer patients were more likely to receive chemotherapy in rural areas of NC than in urban areas, which may be a reflection of solid infrastructure of rural Medicaid services or outreach programs targeting rural rather than urban areas. However, at the same time, chemotherapy likelihood fell in rural areas as distance to the nearest provider increased. Thus, there is a potential for the NC Medicaid to improve guideline-consistent care for SEER-staged III, regional cancer patients in rural areas by removing distance-related obstacles and ensuring viable transportation options exist for patients living more than 20 miles away from the nearest chemotherapy provider.
The descriptive statistics raised an additional interesting point. Both local and regional cancer patients’ actual distances travelled to the chosen chemotherapy facility were longer than the distances to the nearest providers actively serving Medicaid patients. Future research is needed to understand who bypasses the nearest facility and how such a choice is related to the reporting hospital characteristics, willingness of physicians to see Medicaid patients and survival.
While the use of a relatively homogenous sample of Medicaid patients in NC had its advantages for studying the distance-chemotherapy association, its disadvantage is limited generalization of the results across state lines and socio-economic groups of patients. Not being able to geocode addresses and compute the distance to provider for 12% of the sample was also a limitation. We addressed the issue by keeping these observations in the study and accounting for them through a missing value identifier and found a negative association between a missing residential address and the likelihood of receiving chemotherapy in rural areas. We hypothesize that people with missing geocoded addresses quite likely had PO box addresses, which cannot be geocoded. PO boxes are common in less densely populated areas, and thus a missing address may be a proxy for living far from providers. Finally, this study was unable to distinguish between the physician and patient decisions to pursue chemotherapy treatment because we do not know whether the physician made a chemotherapy referral unless the patient actually received the chemotherapy.
Overall, our study has shown that distance to provider can be an obstacle to access to colon cancer care for low income regional cancer patients living in rural areas more than 20 miles from the nearest provider as well as for patients diagnosed with local cancer. Medicaid could increase its guidance-consistent care by relieving travel burdens of regional cancer patients in rural areas. Future research should address why the association between distance and chemotherapy is stronger for local cancer than regional cancer patients and why patients bypass the nearest facilities for adjuvant care.
Acknowledgements
Funding: This manuscript was supported by the American Cancer Society Grant (RSGT-07-011-01-CPHPS) through the generous support of the Edward L. Bakewell, Jr. Charitable Lead Trust. K. Foley was the grant’s principal investigator.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Supplementary
Sample: colon cancer patients who were diagnosed between 1999–2002 (n=1,184)
References
- American Cancer Society (2015) Cancer Facts & Figures 2015. American Cancer Society, Atlanta. Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- Bresalier RS, Kopetz S, Brenner DE. Blood-based tests for colorectal cancer screening: do they threaten the survival of the FIT test? Dig Dis Sci 2015;60:664-71. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8. [Crossref] [PubMed]
- Aarts MJ, Lemmens VE, Louwman MW, et al. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer 2010;46:2681-95. [Crossref] [PubMed]
- Bradley CJ, Given CW, Dahman B, et al. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med 2008;168:521-9. [Crossref] [PubMed]
- Carcaise-Edinboro P, Bradley CJ, Dahman B. Dually eligible and colorectal cancer screening: too little, too late? J Health Care Poor Underserved 2009;20:854-65. [Crossref] [PubMed]
- Polednak AP. Poverty, comorbidity, and survival of colorectal cancer patients diagnosed in Connecticut. J Health Care Poor Underserved 2001;12:302-10. [Crossref] [PubMed]
- Foley KL, Tooze JA, Klepin HD, et al. Adjuvant chemotherapy among medicaid-enrolled patients diagnosed with nonmetastatic colon cancer. Am J Clin Oncol 2011;34:120-4. [PubMed]
- Etzioni DA, El-Khoueiry AB, Beart RW Jr. Rates and predictors of chemotherapy use for stage III colon cancer: a systematic review. Cancer 2008;113:3279-89. [Crossref] [PubMed]
- Luo R, Giordano SH, Zhang DD, et al. The role of the surgeon in whether patients with lymph node-positive colon cancer see a medical oncologist. Cancer 2007;109:975-82. [Crossref] [PubMed]
- Tropman SE, Hatzell T, Paskett E, et al. Colon cancer treatment in rural North and South Carolina. Cancer Detect Prev 1999;23:428-34. [Crossref] [PubMed]
- Aas E. Pecuniary compensation increases participation in screening for colorectal cancer. Health Econ 2009;18:337-54. [Crossref] [PubMed]
- Winget M, Hossain S, Yasui Y, et al. Characteristics of patients with stage III colon adenocarcinoma who fail to receive guideline-recommended treatment. Cancer 2010;116:4849-56. [Crossref] [PubMed]
- Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer 2008;112:909-18.
- Jones AP, Haynes R, Sauerzapf V, et al. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer 2008;44:992-9. [Crossref] [PubMed]
- Scoggins JF, Fedorenko CR, Donahue SM, et al. Is distance to provider a barrier to care for medicaid patients with breast, colorectal, or lung cancer? J Rural Health 2012;28:54-62. [Crossref] [PubMed]
- Huang B, Dignan M, Han D, et al. Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in Kentucky. J Rural Health 2009;25:366-71. [Crossref] [PubMed]
- Celaya MO, Rees JR, Gibson JJ, et al. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (United States). Cancer Causes Control 2006;17:851-6. [Crossref] [PubMed]
- Maheswaran R, Pearson T, Jordan H, et al. Socioeconomic deprivation, travel distance, location of service, and uptake of breast cancer screening in North Derbyshire, UK. J Epidemiol Community Health 2006;60:208-12. [Crossref] [PubMed]
- Cutler DM. The lifetime costs and benefits of medical technology. J Health Econ 2007;26:1081-100. [Crossref] [PubMed]
- McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA 1994;272:859-66. [Crossref] [PubMed]
- Freeman V, Slifkin RT. Rural and urban parents report on access to health case for their children with Medicaid managed care. Working Paper No.84. North Carolina Rural Health Research and Policy, Cecil G. Sheps Center for Health Services Research. UNC Chapel Hill. 2005. Available online: http://www.shepscenter.unc.edu/rural/pubs/report/WP84.pdf
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]