Association between chronic hepatitis C and hepatitis C/HIV co-infection and the development of colorectal adenomas
Introduction
Viruses are infectious particles that have been implicated in the pathophysiology of many diseases, ranging from simple upper respiratory tract infections to malignancies with very poor prognosis. Chronic hepatitis C virus (HCV) infection is one of the leading blood-borne diseases. The incidence of hepatitis C in the United States for the year 2012 was 0.6 per 100,000 people with a total of 21,870 new cases reported (1). HCV is associated with a variety of illnesses including autoimmune vasculitis, clotting deficiencies, lymphoproliferative neoplasm, and solid malignancies (2,3). The relationship between Hepatocellular carcinoma (HCC) and chronic HCV is well established, and there is a growing wealth of knowledge that describes how this virus promotes the development of HCC (4,5). However, very little is known about the effects that this infection can have in the carcinogenesis of other common malignancies, namely colorectal cancers (CRC). Although the majority of CRC arise from adenomatous polyps (6); it is not known if chronic infection with HCV influences the growth and development of these precancerous lesions, promoting CRC.
Historically, HIV/AIDS infection has been associated with many opportunistic malignancies such as Kaposi’s sarcoma; however, since the introduction of highly active antiretroviral therapy (HAART), the incidence of opportunist neoplasm in this population has decreased, along with an increase in life expectancy (7). As a result, the incident of non-AIDS-defining-malignancy (NADM) has increased. Unfortunately, in these patients, such malignancies present at an earlier age and usually follow a more aggressive course (8,9). The exact reasons for this observation are unknown; however, many experts hypothesize that it may be partly due to the profound immune disarrangement produced by the HIV virus in their hosts (10).
The factors that promote the development of colorectal adenoma (CRA) are not as well established as in other neoplasms; namely the correlation between lung cancer and smoking (11,12). The purpose of this study is to explore if there is any relationship between chronic HCV infection and the development of CRA. We will explore if chronic co-infection with HCV and HIV viruses has any synergistic effect in the development of CRA.
Methods
Data was collected from Nassau University Medical Center, a 530-bed tertiary care teaching hospital in East Meadow, New York. Patients who underwent colonoscopy from July 1, 2009 to March 31, 2011 were selected from an electronic data base. A total of 2,146 patients completed colonoscopies during this time. Ninety-five patients were excluded for the following reasons: 17 had inflammatory bowel disease, 12 had malignancy, 22 with prior history of CRA, and 44 were incomplete colonoscopies defined as not achieving cecal intubation.
Hepatitis C was confirmed by one of the following methods: Hepatitis C serological test, HCV RNA by PCR quantitative >200 equivalents/mL, or > HCV RNA 10 equivalents/mL on qualitative tests. HIV testing was performed using rapid HIV testing or screening enzyme immunoassay (EIA) that was later confirmed with western blot. Testing for these viruses was based on risk assessment and clinician’s discretion. A total of 1,183 patients were tested for HCV with 175 having a positive result. A total of 367 patients underwent testing for both viruses of which 47 were positive. Demographics such as age, body mass index (BMI), sex, race, smoking, alcohol, diabetes, hypertension, dyslipidemia, and family history of CRC were collected for all patients in the study and control groups which consisted of those patients testing negative for HCV, and HCV/HIC. Fisher’s exact χ2 test for categorical variables and t-test for continuous variables were used to analyze data between groups. Logistic regression was then performed to obtain odds ratios (OR). SAS 9.3 software was used to perform all statistical analysis with controls set for age, sex, BMI, race, alcohol, and tobacco.
Results
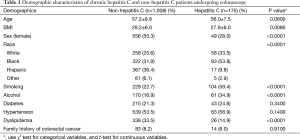
Table 1 displays the demographic characteristics of our HCV (study group) and control groups. A total of 1,183 patients that underwent colonoscopy were tested for HCV. Overall 175 patients were found to be positive for the disease while 1,008 patients tested negative. The study group was male predominant (72%) while the control was female predominant (55.3%). The HCV group consisted of predominantly African American (AA) (53.8%) followed by Caucasian (33.5%), Hispanic (9.8%), and others (2.9%). On the other hand, the control group was composed of Hispanic (36.4%), followed by white (25.6%), black (31.9%), and others (6.1%). There was a higher predominance of smoking in our study group (59.4% vs. 16.9%). In contrast, prevalence of dyslipidemia was higher in the control 33.5% vs. 14.9%. The age, history of diabetes, hypertension, and family history were not significantly different among the control and the study groups. Adenoma detection rate was found to be higher in our study group (26.3% vs. 20.2%), although it did not reach statistical significance. The location, number, and size of the CRA were not statistically different between the control and the study group (Table 2).
Full table

Full table
Table 3 displays the demographic characteristics of our HCV/HIV and control groups. A total of 369 patients who underwent colonoscopy during this time were tested for both viruses. Our study group consisted of 47 patients that tested positive for both. The study group was male predominant (74.5%) while the control was female predominant (54%).The study group was predominantly composed by black patients (65.2%) followed by white (28.3%), Hispanic (4.4%), and others (2.2%). This was statistically different from the control group that was predominantly White (40.4%) followed by Hispanic (30.8%), black (26.7.9%), and others (2.2%). Smoking and alcohol use was more frequently seen in the study than in the control group (57.5% vs. 29.5% and 38.9% vs. 19.3% respectively). On the other hand, there was a higher prevalence of hypertension and dyslipidemia in the control group (55% vs. 38.3% and 34.5% vs. 8.5% respectively). History of diabetes, family history, and age were not significantly different between the two groups. Table 4 shows the results of the HIV/HCV co-infected section of our study. The adenoma detection rate was higher amongst the co-infected group 25.5% vs. 20.8%; however, it did not reach statistical significance. Once more, there was no statistical difference between size, location, or number of CRA between the study and control groups.

Full table

Full table
Discussion
CRC is the third most common malignancy in the United States causing significant morbidity and mortality; most CRC develop from adenomatous polyps (6,13). In 2012, a total of 135,260 people in the United States were diagnosed with this malignancy and 51,783 ultimately succumbed to this disease (13). The relationship between HCV chronic infection and the incidence of CRA is still debated with few studies having explored this question (14). The data regarding whether co-infection with these two viruses has any synergistic effects in the development of these lesions is scarce.
In our paper we found a higher incidence of CRA in HCV patients compared to non-HCV; although, our data failed to reach statistical significance (Table 2). A study conducted by Prakash et al. found similar results. This researcher showed that HCV patients had a higher incidence of CRA detected during screening colonoscopy. Unfortunately, the sample size in this study was small and further conclusions were difficult to draw (15). Our results also agree with another recent retrospective study that found a higher incidence of adenomas in patient with a history of chronic HCV infection (14). The exact mechanism by which chronic HCV infection promotes the development of CRA is not known. However, researchers believe that the carcinogenicity of HCV is likely to be multifactorial. At least in HCC it is thought that malignant transformation is related to the increased number of cell cycles of hepatocytes harboring HCV. Also, it is now established that HCV viral proteins are implicated in the oncogenesis of HCC; namely the HCV core protein. Researchers have shown that this protein can function as a gene regulator and has the ability to inhibit the tumor suppressor gene p53 and induce the transcription factor NF-κB (2,3,9). Studies have shown that overexpression of p53 is associated with more advanced, larger adenomas, villous histology, and high-grade dysplasia (16). Alteration in the NF-κB signaling pathway has been associated with tumorigenesis and malignant progression. In vitro studies have demonstrated that accumulation of cytosolic NF-κB in colon cancer cells correlates with high metastatic potential as well as tumor aggressiveness (17). Both the same mechanisms and others which are not yet known are utilized by HCV on infected colon cells to induce the development of CRA that remains unknown.
The HCV/HIV co-infection data showed a higher incidence of CRA during colonoscopy; but once again it failed to reach statistical significance. Although these viruses are different with regards to cell tropism and the mechanisms that they use to invade and take over the cell’s metabolic machinery, they also share many similarities. Unlike the Herpes virus, which typically infects cells and has periods of active viral replication alternating with periods of very low activity; both HIV and HCV are characterized by sustained viremia once they have invaded the host. Through different mechanisms both viruses interfere with the production of interferon alpha (IFN-α), a key cytokine needed to prevent viral replication and spread. By disrupting proper cytokine production, they produce an unfavorable environment that prevents adequate maturation of dendritic cells which are the main antigen presenting cells (APC) for CD4+ T-helper cells. T-helper cells are responsible for orchestrating an effective immune response that will ultimately result in viral clearance. By preventing appropriate interactions between APC and T-helper cells, these viruses are able to perpetuate viral replication and produce a state of anergy and immunotolerance (10). Thus, we theorize that since these two viruses share so many similar pathological mechanisms it is not unreasonable to speculate that they may share oncogenic properties that could act in a synergistic manner and promote the development of CRA.
One of the main limiting factors of our research was the small size of our study populations. As a single institution study performing a retrospective investigation, we were limited as well. The selection process which was rather arbitrary may also have a negative impact in our results. Finally, although we adjusted for variables such as age, sex, etc., we suspect that the negative outcome may also be secondary to other independent factors that may influence the development of CRA in patients with HCV and with co-infection. However, our paper clearly shows an increase in incidence of CRA found during colonoscopy in patients with HCV infection compared to the control group, and that co-infection with these two viruses may increase the incidence of these pre-cancerous lesions through a mechanism yet to be described.
Conclusions
A higher detection rate of CRP was seen in the HCV population; however, it failed to reach statistical significance. Whether co-infection with HIV/HCV increases the incidence of CRA and/or has a synergistic effect remains to be determined. However, our results raise the question of whether HCV and HIV/HCV co-infected patients will benefit from screening colonoscopy at an earlier age. This issue merits further investigation in a large, multi-center prospective study because once these patients have developed CRC the treatment is greatly hindered by their underlying co-morbidities and the interactions between chemotherapeutic drugs and their anti-viral treatments (18,19).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee (No. 11-068B).
References
- Centers for Disease Control and Prevention. Viral Hepatitis Surveillance – United States, 2012. Last updated August 20, 2014. Accessed on April 25, 2014. Available online: http://www.cdc.gov/hepatitis/Statistics/index.htm
- Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med 1995;123:615-20. [Crossref] [PubMed]
- El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology 2002;36:1439-45. [Crossref] [PubMed]
- Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene 2006;25:3834-47. [Crossref] [PubMed]
- Moriya K, Fujie H, Shintani Y, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med 1998;4:1065-7. [Crossref] [PubMed]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-67. [Crossref] [PubMed]
- Miiro G, Todd J, Mpendo J, et al. Reduced morbidity and mortality in the first year after initiating highly active anti-retroviral therapy (HAART) among Ugandan adults. Trop Med Int Health 2009;14:556-63. [Crossref] [PubMed]
- Nguyen ML, Farrell KJ, Gunthel CJ. Non-AIDS-defining malignancies in patients with HIV in the HAART era. Curr Infect Dis Rep 2010;12:46-55. [Crossref] [PubMed]
- Silverberg MJ, Abrams DI. Do antiretrovirals reduce the risk of non-AIDS-defining malignancies? Curr Opin HIV AIDS 2009;4:42-51. [Crossref] [PubMed]
- Ng CT, Snell LM, Brooks DG, et al. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe 2013;13:652-64. [Crossref] [PubMed]
- Pearl R. Tobacco smoking and longevity. Science 1938;87:216-7. [Crossref] [PubMed]
- The health consequences of smoking—50 years of progress: a report of the surgeon general. Available online: http://ash.org/wp-content/uploads/2014/01/full-report.pdf
- U.S. Cancer Statistics Working Group. United States cancer statistics: 1999–2011 incidence and mortality web-based report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2015.
- Rustagi T, Zarookian EI, Qasba O, et al. Chronic hepatitis C as a risk factor for colorectal adenoma. Int J Colorectal Dis 2014;29:75-80. [Crossref] [PubMed]
- Prakash R, Shah N, Mullen K. Chronic hepatitis C patients have larger colonic adenomas. Am J Gastroenterol 2009;104:S138.
- Einspahr JG, Martinez ME, Jiang R, et al. Associations of Ki-ras proto-oncogene mutation and p53 gene overexpression in sporadic colorectal adenomas with demographic and clinicopathologic characteristics. Cancer Epidemiol Biomarkers Prev 2006;15:1443-50. [Crossref] [PubMed]
- Simiantonaki N, Kurzik-Dumke U, Karyofylli G, et al. Loss of E-cadherin in the vicinity of necrosis in colorectal carcinomas: association with NFkappaB expression. Int J Oncol 2007;31:269-75. [PubMed]
- Melisko ME, Fox R, Venook A. Reactivation of hepatitis C virus after chemotherapy for colon cancer. Clin Oncol (R Coll Radiol) 2004;16:204-5. [Crossref] [PubMed]
- Spano JP, Costagliola D, Katlama C, et al. AIDS-related malignancies: state of the art and therapeutic challenges. J Clin Oncol 2008;26:4834-42. [Crossref] [PubMed]


