The prognostic role of lactate dehydrogenase serum levels in patients with hepatocellular carcinoma who are treated with sorafenib: the influence of liver fibrosis
Introduction
Sorafenib, a multi-kinase inhibitor, is an effective anticancer drug for patients with advanced hepatocellular carcinoma (HCC). Its effectiveness is based on activity against Raf-1, B-Raf, vascular endothelial growth factor (VEGF) receptor-2, platelet-derived growth factor (PDGF) receptors and c-kit receptors, which have antiangiogenic effects (1-3). Although several high-level randomized studies showed that sorafenib improved survival of patients with unresectable HCC, a prediction of efficacy for individual patients is difficult (1,4). Recently, Faloppi et al. reported that the pretreatment serum lactate dehydrogenase (LDH) level is a useful parameter to assess the prognosis of HCC patients treated with sorafenib (5). A similar predictive role for LDH was found in renal cell carcinoma, rectal carcinoma, small cell lung cancer and pancreatic cancer patients treated with sorafenib (6-9). However, Sacco et al. found that baseline LDH levels had no correlation with the prognosis of HCC patients treated with sorafenib (10). These controversial results have not been clearly explained.
The idea that LDH could be a prognostic factor for patients who undergo anticancer treatment is based on evidence that hypoxic circumstances in tumors may promote cancer development, and serum LDH levels could reflect the degree of intra-tumor hypoxia (11,12). For HCC, which commonly develops in patients with liver cirrhosis, there is an aggressive increase in tumors and there is also liver fibrosis, which both cause intrahepatic hypoxia (13-16). Serum LDH levels in HCC patients, thus, reflect both the tumor aggressiveness and the degree of liver fibrosis. Therefore, investigation into the correlation between serum LDH levels and the prognosis of the patients with HCC requires consideration of background liver fibrosis, which could potentially explain the conflicting results observed for the use of serum LDH as a prognostic factor.
In addition to baseline LDH levels, serum LDH levels increase during the sorafenib treatment, which has not been well examined. Sorafenib increases systemic vascular resistance and it suppresses tumor angiogenesis via inhibition of the VEGF signaling pathway. This is supported by the observation that many patients treated with sorafenib experience an increase in blood pressure (2,17). Inhibition of VEGF signaling decreases endothelial NO synthase (eNOS) expression, causing vascular smooth muscle cell constriction and inducing endothelial cell apoptosis (2). Because existing liver fibrosis produces hypoxic circumstances in the liver, administration of sorafenib to patients with cirrhosis could further deteriorate hepatic functional reserve. Although acute liver failure caused by sorafenib has been only rarely reported, additional cases may have been missed. Sorafenib has been used only for patients who have advanced-stage disease, and liver failure resulting from adverse effects related to sorafenib might have been considered a progression of the original disease.
In this study, we aimed to investigate the correlation between the early response to sorafenib in patients with HCC and pretreatment liver function, including serum LDH levels and fibrotic parameters. In earlier reports, the effect of sorafenib was evaluated using overall survival and/or progression free survival (5,10). However, because the majority of the patients develop drug-resistance, early response was considered a more appropriate end point of the observation.
Methods
This study was designed as a prospective multi-institutional joint investigation. Consecutive patients with advanced unresectable HCC and hepatic functional reserve, who were classified as Child-Pugh A, were enrolled into the study. HCC diagnosis was confirmed using computed tomography (CT) within 1 month before the start of sorafenib treatment. The HCC stage was determined using the Japan Integrated Staging Score (JIS score) based on CT results (18). From August 2012 to March 2015, 89 patients were enrolled. They comprised 77 males and 12 females, aged 34 to 87 years, with a mean age of 70.3 years. Sorafenib treatment at a dose of 400 mg/day, which was decreased to 200 mg/day when adverse effects greater than grade 2 were observed, was started. Serum tests for hepatic function and blood cell counts were performed at the beginning of treatment, and every 2 weeks thereafter. Among the enrolled patients, five discontinued sorafenib within less than 4 weeks because of severe adverse effects greater than grade 3, including one event of general fatigue and one event of appetite loss, and the other three were events of deteriorated hepatic function.
Early tumor response to sorafenib was judged based on CT results 3 months after the start of sorafenib treatment in patients who continued sorafenib administration for more than 4 weeks. The tumor response was classified as progressive disease (PD), stable disease (SD), partial response (PR) and complete response (CR), according to the modified response evaluation criteria in solid tumors (19).
The degree of fibrosis was estimated using the aspartate aminotransferase (AST) to platelet ratio index (APRI), as previously described; patients with a value of more than 0.7 were considered to have significant liver fibrosis, and those with a value of more than 1.0 were considered to have severe fibrosis (20).
This study was approved by the Kyushu University Hospital Ethics Committee, and informed consent was obtained from the patients.
Statistical analysis
Laboratory results are presented as the mean ± standard deviation (SD) for quantitative measurements. Differences in the mean values of quantitative variables were examined using the Tukey-Kramer honestly significant difference test, and those of non-quantitative variables were tested using Pearson’s Chi-squared test. To assess the early response effect of sorafenib, laboratory variables were analyzed using a univariate logistic analysis of PD vs. SD/PR/CR, which was followed by a multivariate logistic regression analysis using stepwise variable selection after excluding the factors with a P value more than 0.10 in the univariate logistic analysis.
Results
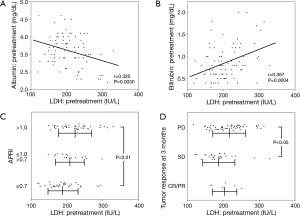
In the 84 patients who received sorafenib continuously for more than 4 weeks, an early response of PD at 3 months was seen in 52 patients, SD in 26 patients, PR in 5 patients and CR in 1 patient (Table 1). Pretreatment laboratory results showed that serum LDH levels were positively correlated with those of albumin and negatively correlated with those of bilirubin (Figure 1). Compared with APRI, the mean serum LDH level was higher in patients with a high APRI score, while the HCC tumor stage did not influence serum LDH levels. Patients in the PD group had the highest mean LDH serum level for both pretreatment and early response.

Full table
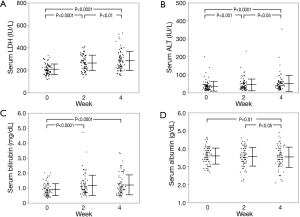
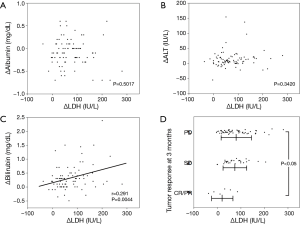
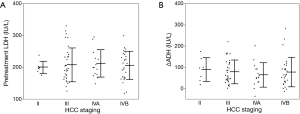
Serum test results revealed that leakage enzymes such as AST, alanine transaminase (ALT), alkaline phosphatase (ALP) and LDH gradually increased (Figure 2). The mean degree of change in serum LDH levels (ΔLDH) at 0, 2 and 4 weeks was 207.0, 264.2 and 281.6 IU/L, respectively, which was higher than that of ALT (ΔALT; 37.0, 45.0 and 50.9 IU/L at 0, 2 and 4 weeks, respectively). Hepatic functional reserve factors were tested and the decrease in serum albumin (Δalbumin) was minimal, while the increase in bilirubin (Δbilirubin) was more than 0.5 mg/dL at 4 weeks in 19 of 84 patients. ΔLDH correlated weakly with Δbilirubin at 4 weeks, but it had no correlation with Δalbumin or ΔALT (Figure 3). The influence of ΔLDH on the early response to sorafenib was also examined. Although patients with PR or CR had lower ΔLDH, there was no significant difference between PD and SD. The correlation between HCC stage and pretreatment LDH or ΔLDH was analyzed, and there were no differences at any stage (Figure 4).
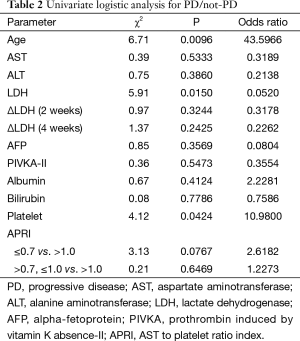
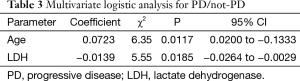
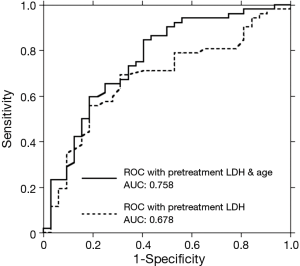
To evaluate the influence of variables on the early response, a univariate logistic analysis of PD versus non-PD was performed (Table 2). Age, pretreatment LDH level, platelet count and APRI had a p value less than 0.1. Subsequent stepwise analysis selected age and pretreatment LDH as independent prognostic factors. Multivariate logistic analysis demonstrated that a younger age and higher pretreatment LDH levels are associated with a worse early response to treatment with sorafenib (Table 3). A receiver operating characteristic (ROC) curve drawn with both parameters had an area under the curve (AUC) of 0.758, while that with the single parameter of pretreatment LDH level was 0.678 (Figure 5).
Full table
Full table
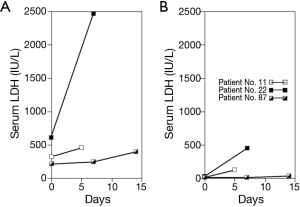
The three patients who stopped sorafenib treatment because of acute liver failure all showed a distinct increase in serum LDH levels just before the symptoms appeared. Common characteristics included marked elevation of serum LDH compared with the change in serum ALT (Figure 6). Additionally, these patients had reduced portal flow volume; two patients had a tumor thrombus in the portal trunk, and one patient had tumors compressing the main portal branches. Their serum ALP and γ-glutamyl transferase levels remained at relatively low levels. None of these patients had a high fever, rash, lymphadenopathy or eosinophilia. These findings suggest that intrahepatic hypoxia mainly promoted the progression of liver failure. Although one patient recovered to the baseline hepatic function after cessation of sorafenib, two patients progressed to liver failure and died after 5 and 37 days.
Discussion
LDH is a key enzyme that catalyzes the conversion of pyruvate to lactate in hypoxic conditions and it is up-regulated under a hypoxic circumstance (21). Thus, an increase in serum LDH levels could be a marker for various hypoxic conditions in the body. Recently, many authors reported the usefulness of serum LDH levels as a prognostic marker for various solid tumors including HCC (6-9). They evaluated the role of LDH, assuming that malignant cells in a tumor would have a low oxygen supply and would be a main source of increased LDH. For HCC, however, most patients have liver cirrhosis, which can cause intrahepatic hypoxia. To evaluate the prognostic role of LDH for patients with HCC, the source of the LDH that is produced (i.e., from the tumors or the fibrotic whole liver) must be clarified.
Baseline LDH was tested and we found that LDH levels positively correlated with bilirubin levels and APRI, and negatively correlated with albumin levels. These results suggest that baseline LDH levels were influenced by the degree of liver fibrosis. Although all patients enrolled into this study were classified as Child-Pugh class A, there can be a relatively wide range of liver fibrosis in these patients. However, baseline LDH levels did not correlate with the HCC stage, and baseline LDH levels seem to more strongly depend on hypoxia from the whole fibrotic liver rather than on hypoxia resulting from tumors.
After administration of sorafenib, there was a rapid increase in LDH levels, but only a small increase in ALT levels, and ΔLDH was not correlated with ΔALT. In addition, response to sorafenib treatment did not correlate with ΔLDH. These results indicate that the increase in LDH that resulted from sorafenib could be caused by progression of whole-liver hypoxia, not by the amount of damaged or proliferating malignant cells.
In patients with liver cirrhosis, oxygen delivery to hepatocytes is generally disturbed by intrahepatic shunts or capillarization of the sinusoids, and systemic vessel muscle constriction caused by sorafenib would worsen such a hypoxic situation (13-16). Both pretreatment LDH and ΔLDH were dependent on the degree of liver fibrosis, but we found no correlation between them. We suggest that the patients enrolled into this study were classified as Child-Pugh class A (in which a marked decrease of portal flow volume is rare), but these patients had sufficient portal venous blood supply to avoid high levels of hypoxia during sorafenib treatment. If we had widened the eligibility criteria to Child-Pugh class B/C, there may have been a correlation between pretreatment LDH and ΔLDH.
The three patients who stopped receiving sorafenib within less than 4 weeks because of liver failure had their portal vein obstructed by a tumor thrombus or by tumors compressing the portal vein, which should cause a decrease in portal flow. Each of these patients had an unusual increase in serum LDH levels compared with serum ALT levels, suggesting that there are high levels of whole-liver hypoxia. We emphasize that systemic vessel constriction caused by sorafenib contributed to the decreased portal flow volume, which can cause fatal liver failure. Although Van Hootegem et al. suggested the possibility that acute liver failure caused by sorafenib might result from drug-induced hypersensitivity syndrome (DIHS), none of our patients had the characteristic symptoms of DIHS such as fever, rash, lymphadenopathy or eosinophilia (22). To the best of our knowledge, most HCC patients who were treated with sorafenib who had CR had portal vein obstruction, which suggests that, as far as the hepatic functional reserve permits, sorafenib could contribute to production of sufficiently hypoxic conditions in the tumor to cause complete necrosis when there is decreased portal flow volume. Thus, the use of sorafenib for HCC patients with reduced portal flow volume could cause severe hypoxia in some hepatic lesions, which subsequently might produce complete remission, and/or induce liver failure when the patient has poor hepatic functional reserve.
Multivariate analysis showed that pretreatment LDH and age were independent predictive factors for a drug-response at 3 months while ΔLDH had no correlation with the therapeutic response. Our results could not be directly compared with earlier studies because the earlier studies evaluated the prognostic role of serum LDH levels in correlation with overall survival and progression free survival. However, our data are consistent with the result of Faloppi’s group, who demonstrated that there was a better prognosis in patients with lower levels of pretreatment serum LDH (5). If the increased serum LDH levels reflect whole liver hypoxia, as described above, and such circumstances decrease the effect of sorafenib, we hypothesize that a pretreatment hypoxic situation could increase tumor aggressiveness or promote drug resistance. Recently, using mouse models of primary liver tumors, Bogaerts et al. showed that hypoxia in advanced stages resulted in increased expression of liver progenitor cell characteristics that indicate a poor outcome (23). Liu et al. demonstrated that the targeted knock-down of hypoxia-inducible factor-2α, which is associated with cell proliferation under hypoxic conditions, in combination with sorafenib, markedly decreased proliferation in HCC cells (24). This supports the possibility that background hypoxia caused by liver fibrosis has detrimental effects on patients treated with sorafenib.
It is unknown why age was selected as a prognostic factor for patients receiving sorafenib treatment. The average age of patients enrolled into this study was relatively high compared with previous reports evaluating the effect of sorafenib for HCC patients, and no other studies found that the patient’s age was a prognostic factor. Although Di Costanzo et al. showed that older patients (≥70 years old) had a better prognosis compared with younger patients, but the difference was not statistically significant (25). They suggest the possibility that, because of the characteristics of their vasculature, older patients might be more susceptible to the antiangiogenic effect of sorafenib. Because the amount of elderly patients with HCC should increase in the future as a result of improvements in anti-viral drugs for hepatitis B and C and in treatments for cirrhotic patients, the effect of sorafenib on an aging population needs to be clarified.
In this study, we showed that the baseline serum LDH levels in HCC patients treated with sorafenib correlated with the degree of liver fibrosis, and that this could be a marker for early response to sorafenib. A marked increase of serum LDH levels during administration of sorafenib might indicate subsequent acute liver failure, especially in patients with deteriorated portal flow volume. Close observation of serum LDH levels before and during treatment with sorafenib would be useful in management of patients who are receiving this therapy.
Acknowledgements
The authors would like to thank Ms. Keiko Nishimura for assistance with manuscript preparation.
This study was supported by research funding from Beyer Yakuhin, Ltd. (Osaka).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Kyushu University Hospital Ethics Committee, and informed consent was obtained from the patients.
References
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Humphreys BD, Atkins MB. Rapid development of hypertension by sorafenib: toxicity or target? Clin Cancer Res 2009;15:5947-9. [Crossref] [PubMed]
- Jain L, Sissung TM, Danesi R, et al. Hypertension and hand-foot skin reactions related to VEGFR2 genotype and improved clinical outcome following bevacizumab and sorafenib. J Exp Clin Cancer Res 2010;29:95. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Faloppi L, Scartozzi M, Bianconi M, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer 2014;14:110. [Crossref] [PubMed]
- Kubackova K, Bortlicek Z, Pavlik T, et al. Prognostic factors in renal cell carcinoma patients treated with sorafenib: results from the Czech registry. Target Oncol 2015;10:385-92. [Crossref] [PubMed]
- Scartozzi M, Giampieri R, Maccaroni E, et al. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 2012;106:799-804. [Crossref] [PubMed]
- Hermes A, Gatzemeier U, Waschki B, et al. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer - a retrospective single institution analysis. Respir Med 2010;104:1937-42. [Crossref] [PubMed]
- Faloppi L, Bianconi M, Giampieri R, et al. The value of lactate dehydrogenase serum levels as a prognostic and predictive factor for advanced pancreatic cancer patients receiving sorafenib. Oncotarget 2015;6:35087-94. [PubMed]
- Sacco R, Mismas V, Granito A, et al. Correlation between LDH levels and response to sorafenib in HCC patients: an analysis of the ITA.LI.CA database. Int J Biol Markers 2015;30:e65-72. [Crossref] [PubMed]
- Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol 2000;35:71-103. [Crossref] [PubMed]
- Maxwell PH, Dachs GU, Gleadle JM, et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A 1997;94:8104-9. [Crossref] [PubMed]
- Rosmorduc O, Wendum D, Corpechot C, et al. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol 1999;155:1065-73. [Crossref] [PubMed]
- Corpechot C, Barbu V, Wendum D, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 2002;35:1010-21. [Crossref] [PubMed]
- Bozova S, Elpek GO. Hypoxia-inducible factor-1alpha expression in experimental cirrhosis: correlation with vascular endothelial growth factor expression and angiogenesis. APMIS 2007;115:795-801. [Crossref] [PubMed]
- Van Steenkiste C, Ribera J, Geerts A, et al. Inhibition of placental growth factor activity reduces the severity of fibrosis, inflammation, and portal hypertension in cirrhotic mice. Hepatology 2011;53:1629-40. [Crossref] [PubMed]
- Li Y, Li S, Zhu Y, et al. Incidence and risk of sorafenib-induced hypertension: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2014;16:177-85. [Crossref] [PubMed]
- Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003;38:207-15. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726-36. [Crossref] [PubMed]
- Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem 1995;270:21021-7. [Crossref] [PubMed]
- Van Hootegem A, Verslype C, Van Steenbergen W. Sorafenib-induced liver failure: a case report and review of the literature. Case Reports Hepatol 2011;2011:941395.
- Bogaerts E, Heindryckx F, Devisscher L, et al. Time-dependent effect of hypoxia on tumor progression and liver progenitor cell markers in primary liver tumors. PLoS One 2015;10:e0119555. [Crossref] [PubMed]
- Liu F, Dong X, Lv H, et al. Targeting hypoxia-inducible factor-2α enhances sorafenib antitumor activity via β-catenin/C-Myc-dependent pathways in hepatocellular carcinoma. Oncol Lett 2015;10:778-784. [PubMed]
- Di Costanzo GG, Tortora R, De Luca M, et al. Impact of age on toxicity and efficacy of sorafenib-targeted therapy in cirrhotic patients with hepatocellular carcinoma. Med Oncol 2013;30:446. [Crossref] [PubMed]


