
C-reactive protein is a predictor of severe infective complications following gastrectomy—a retrospective analysis
Highlight box
Key findings
• C-reactive protein (CRP) is a strong negative predicter of severe infective complications following gastrectomy.
What is known and what is new?
• CRP can be used in the post-operative period to help predict post-operative complications.
• Our research suggests CRP can be predictive as early as post-operative day 2 for this patient cohort.
What is the implication, and what should change now?
• CRP values may be useful in prompting early investigation or facilitating safer, earlier discharge from hospital. Health services may benefit by determining similar cut-offs based on their own unique patient populations.
Introduction
Post-operative complications following gastrectomy have severe consequences for patients in terms of length of stay, overall mortality, and long-term health related quality of life (1-3). There is a strong correlation between post-operative morbidity and poorer cancer specific survival (4,5). Furthermore, post-operative complications are strongly associated with increased costs of care (6). The literature demonstrates that prolonged length of stay in hospital is associated with an increased risk of nosocomial infection (7). Accordingly, safely discharging low risk patients earlier from hospital has multiple potential benefits.
Simple methods for predicting and excluding developing complications are likely to be useful. If successfully validated, this tool may assist clinicians to recognise and treat developing complications more swiftly and help identify patients who are unlikely to develop a complication. Identifying patients who can be safely discharged ‘early’ reduces the burden on hospital resources and economic pressures (8). A clinically useful tool to predict morbidity will help contribute to a safer and more efficient healthcare system.
C-reactive protein (CRP) is a non-specific inflammatory marker produced in the liver in response to interleukin-6 released from macrophages and T cells (9). It functions by binding to receptors on cells to initiate the complement system (9,10). Ultimately, this promotes the ingestion and destruction of these cells (9). There is a well-established association between higher levels of CRP and a mounting inflammatory response (10-12). Therefore, it is reasonable that CRP may have a role in predicting post-operative complications (13).
CRP has shown some capacity to predict complications following gastrectomy (14-21). Despite this, there is discrepancy in the diagnostic accuracy and optimum cut-offs (14-21). Furthermore, published literature examining CRP post-gastrectomy does not remove patients from the analysis following the diagnosis of their most severe complication. This may skew the true diagnostic accuracy of CRP and thus the reliability of the test applied in clinical practice. Evaluation of the daily change in CRP for predicting complications for this cohort has not been extensively examined. This was adopted with the hypothesis that this would better reflect the underlying physiological process on a day-to-day basis, rather than a cumulative basis. Given that CRP is a cheap, minimally invasive and an easy test to perform, expanding its diagnostic utility is likely to be beneficial. We present the article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-675/rc) (22).
Methods
Participants
Patients who had an elective or emergency gastrectomy for the treatment of gastric malignancy at a tertiary referral centre between June 2014 and April 2021 were included in this study. Participants were excluded if the operation was performed with palliative intent, they were under 18 at the time of surgery, or their surgery was abandoned because of the inoperable nature of the cancer.
Data collection
Data points were collected and prospectively maintained using the REDCap electronic platform, hosted at the University of Melbourne (23,24). Deidentified parameters included gender, age at time of surgery, body mass index (BMI), Charlson Comorbidity Index, type of surgery performed, method of access, operation time and total blood loss during the operation. Details regarding the histopathology were also collected. CRP levels were recorded on the day of surgery and for the following 7 days when available. Post-operative complications, the post-operative day (POD) of diagnosis, Clavien-Dindo gradings, the 30- and 90-day mortality rates were also contemporaneously recorded (25). Other measures of morbidity such as unplanned intensive care unit (ICU) admission and readmission within 30 days were included.
Complications were defined based on the documentation by the treating team. Complications with a Clavien-Dindo Grading ≥3a were classified as severe (25). They were further divided into infective and non-infective. Infective complications included all forms of sepsis. This includes pneumonia, genitourinary, wound infection, biliary sepsis, abdominal sepsis, mediastinitis, duodenal stump leaks and anastomotic leaks. Non-infective complications included atelectasis, pneumothorax, pleural effusion, cardiac arrest, myocardial infarction, dysrhythmia, heart failure, hypoxia, nausea, subcutaneous emphysema, acute kidney injury, ileus, pancreatitis, hernia, deep vein thrombosis, pulmonary embolus, stroke and haemorrhage.
Statistical analysis
A retrospective analysis examining the association between CRP following gastrectomy and post-operative complications was performed using STATA 16.1 (StataCorp, College Station, TX, USA). CRP was the independent variable. The dependent variables included complications, unplanned ICU admission and readmission within 30 days.
The predictive capacity of CRP for each POD was assessed with receiver operator characteristic (ROC) analysis. These curves were compared using the area under the curve (AUC) with a confidence interval of 95% determined using the DeLong approach. The AUC serves as a measure of diagnostic accuracy. An AUC with a statistically significant result >0.500 was considered as having diagnostic usefulness (26). These PODs were subsequently analysed using the Youden’s index (27). This statistical method determines the optimum cut-off based on the sum of sensitivity and specificity of each discrete value in the data set. Further analysis was performed to assess the positive (PPV) and negative predictive values (NPV) of these cut-offs. This method was also applied to unplanned ICU admission and readmission.
Our method omitted patients from the study on subsequent PODs following diagnosis of their most severe complication. This assists in removing confounding factors which influence CRP as either sepsis initially worsens or as complications are treated. This was adopted with the hypothesis it would better reflect the ability of CRP to predict complications. Ultimately, this would assist clinicians to make better informed decisions throughout the post-operative period.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients were consented for data collection and subsequent analysis. The study was reviewed and approved by the St Vincent’s Hospital Melbourne Human Research Ethics Committee (HREC), which is registered with the National Health and Medical Research Council (No. HREC/16/SVHM/127). The full protocol can be accessed by contacting the corresponding author.
Results
General
Sixty patients, 41 (68%) males and 19 (32%) females were included; 34 (57%) patients had a partial gastrectomy, 20 (33%) patients had a total gastrectomy, 6 (10%) had an extended total gastrectomy. Of the total cohort, 34 (57%) patients experienced a post-operative complication; 11 (18%) patients were specifically diagnosed with a severe infective (SI) complication (Table 1). The SI complications comprised of four patients with pneumonia, three patients with abdominal sepsis, two patients with esophagoenteric leaks, one patient with a duodenal stump leak and one patient with genitourinary sepsis requiring dialysis; 20 (33%) patients had multiple complications; 11 (18%) patients were readmitted within 30 days and 4 (7%) had unplanned ICU admissions. One patient died within 30 days (2%) and 3 within 90 days (5%). Given that CRP is synthesised in the liver, no participants had a pre-operative diagnosis of liver cirrhosis or impaired liver function (9).
Table 1
| Demographical and clinical information | All patients | No complications | Severe infective | Overall complications |
|---|---|---|---|---|
| Number, n [%] | 60 [100] | 26 [43] | 11 [18] | 34 [57] |
| Male | 41 [68] | 17 [65] | 8 [73] | 24 [71] |
| Female | 19 [32] | 9 [35] | 3 [27] | 10 [29] |
| Age, mean ± SD (years) | 65±15 | 58±18 | 73±9 | 71±11 |
| BMI, mean ± SD (kg/m2) | 26±5 | 25±5 | 28±4 | 27±5 |
| Charlson Comorbidity Index, n [%] | ||||
| 0–3 | 12 [20] | 9 [35] | 4 [36] | 3 [9] |
| 4–7 | 45 [75] | 16 [62] | 5 [45] | 29 [85] |
| 8–10+ | 3 [5] | 1 [4] | 2 [18] | 2 [6] |
| Type of operation, n [%] | ||||
| Partial | 34 [57] | 18 [69] | 6 [55] | 16 [47] |
| Total | 20 [33] | 7 [27] | 2 [18] | 13 [38] |
| Extended total | 6 [10] | 1 [4] | 3 [27] | 5 [15] |
| Method of access, n [%] | ||||
| Open | 42 [70] | 17 [65] | 10 [91] | 25 [74] |
| Laparoscopic | 18 [30] | 9 [35] | 1 [9] | 9 [26] |
| Total operative time, mean ± SD (min) | 309±103 | 290±60 | 349±142 | 334±123 |
| Total blood loss, mean ± SD (mL) | 209±163 | 142±116 | 309±156 | 261±176 |
| Tumour type, n [%] | ||||
| Adenocarcinoma | 44 [73] | 17 [65] | 10 [91] | 27 [79] |
| Neuroendocrine | 2 [3] | 1 [4] | 0 [0] | 1 [3] |
| Other | 14 [23] | 8 [31] | 1 [9] | 6 [18] |
| CRP (mg/mL), median [range] [n] | ||||
| POD DOS | 33 [12–218] [11] | 36 [12–109] [6] | 218 [218–218] [1] | 14 [12–218] [5] |
| POD 1 | 75 [8–178] [42] | 75 [15–158] [21] | 97 [32–178] [8] | 74 [8-178] [21] |
| POD 2 | 167 [24–354] [47] | 106 [24-207] [21] | 197 [155–354] [8] | 195 [80–354] [26] |
| POD 3 | 168 [41–387] [38] | 109 [41–237] [21] | 242 [111–387] [7] | 259 [111–387] [17] |
| POD 4 | 118 [17–364] [43] | 77 [17–219] [24] | 236 [72–335] [8] | 211 [72–364] [19] |
| POD 5 | 99 [20–404] [36] | 62 [20–190] [21] | 144 [58–404] [9] | 144 [58–404] [15] |
| POD 6 | 78 [24–336] [31] | 48 [24–145] [19] | 150 [69–251] [7] | 152 [63–336] [12] |
| POD 7 | 57 [16–368] [29] | 51 [16–170] [18] | 116 [54–351 [8] | 114 [54–368] [11] |
BMI, body mass index; SD, standard deviation; CRP, C-reactive protein; POD, post-operative day; DOS, day of surgery.
Predicting complications
POD 2 was the earliest day following surgery which demonstrated significant results for predicting SI complications following gastrectomy. An optimal cut-off of 180 mg/L (AUC: 0.789, 95% CI: 0.636–0.941) achieved a sensitivity and specificity of 87.50% and 66.67% respectively. It also demonstrated a PPV of 35.00% but a NPV of 96.30% (Table 2 and Figure 1). The ΔCRP only demonstrated significance between POD 1 and 2 (AUC: 0.863, 95% CI: 0.727–0.999). An increase of 64 mg/L between these days resulted in a PPV and NPV of 37.50% and 100.00% respectively.
Table 2
| POD | AUC (95% CI) | Cut-off (mg/L) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Severe infective complications—daily CRP | ||||||
| POD 2 | 0.789 (0.636–0.941) | 180 | 87.50 | 66.67 | 35.00 | 96.30 |
| POD 3 | 0.786 (0.606–0.956) | 200 | 85.71 | 67.74 | 37.50 | 95.45 |
| POD 4 | 0.796 (0.623–0.971) | 131 | 87.50 | 71.43 | 41.18 | 96.15 |
| POD 5 | 0.815 (0.658–0.976) | 106 | 88.89 | 74.07 | 53.34 | 95.24 |
| POD 6 | 0.770 (0.588–0.948) | 63 | 100.00 | 54.17 | 38.89 | 100.00 |
| POD 7 | 0.824 (0.666–0.983) | 108 | 62.50 | 90.48 | 71.43 | 86.36 |
| Overall complications—daily CRP | ||||||
| POD 2 | 0.858 (0.751–0.965) | 167 | 76.92 | 85.71 | 86.96 | 75.00 |
| POD 3 | 0.947 (0.880–1.000) | 176 | 94.18 | 90.47 | 88.89 | 95.00 |
| POD 4 | 0.900 (0.810–0.991) | 131 | 78.95 | 91.67 | 88.24 | 84.62 |
| POD 5 | 0.864 (0.740–0.994) | 102 | 86.67 | 80.95 | 76.47 | 89.47 |
| POD 6 | 0.868 (0.743–0.994) | 49 | 100.00 | 63.16 | 63.18 | 100.00 |
| POD 7 | 0.864 (0.732–0.995) | 51 | 100.00 | 61.11 | 61.11 | 100.00 |
AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; CRP, C-reactive protein; POD, post-operative day.
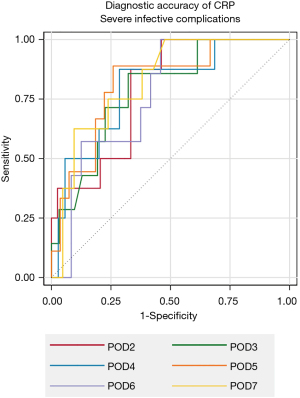
Overall complications were best predicted on POD 3 with an optimal cut-off of 176 mg/L (AUC: 0.947, 95% CI: 0.880–1.000). This resulted in a PPV and NPV of 88.89% and 95.00% respectively (Table 2). The ΔCRP between POD 2 and 3 were the result that demonstrated significant findings (AUC: 0.684, 95% CI: 0.502–0.866). An increase of 23 mg/L between these days resulted in a PPV of 69.23% and NPV of 68.18%.
Readmission and unplanned ICU
Readmission within 30 days was generally not well predicted by CRP. Significant results were only demonstrated on POD 2 (AUC: 0.791, 95% CI: 0.643–0.939). The Youden Index revealed a cut-off of 180 mg/L yielding a sensitivity of 88.89% and specificity of 68.42%. This was accompanied by a PPV and NPV of 40.00% and 96.30% respectively. No POD demonstrated significant results in predicting unplanned ICU admission.
Discussion
This series presents data supporting CRP as a simple test which can assist in guiding early investigations and with a high accuracy, predicting the absence of SI complications following gastrectomy. Based on this data we recommend CRP as a useful supplemental tool to assist with clinical decision making in the post-operative period following gastrectomy. In particular, the strong NPVs early in the post-operative course implies that CRP may have a supportive role in expediting discharge in some patients and thus reduce length of stay and associated issues (6).
CRP has previously been reported as a useful negative predictor of complications following gastrectomy (15,17,19). The diagnostic accuracy and optimum cut-off result reported in this series is similar to other published literature. Shishido et al. reported a cut-off of 177 mg/L on POD 3 as optimal for predicting SI complications (19). Similarly, Zhang et al. found a cut-off of 172 mg/L on POD 3 was best at predicting major infective complications following laparoscopy-assisted gastrectomy (14). Individual studies are likely to show variance according to different patient cohorts, surgical techniques and other management protocols. As such, to best use CRP as a discharge tool, individual institutions should assess their own local data to evaluate local cut off values. Notably, our method of omitting patients from the study following diagnosis of their most severe complication did not seem to have an appreciable numerical difference to the final values compared to these studies (14,15,17,19). Our results demonstrated significance as early as POD 2. This suggests CRP may have a role in earlier detection in post-operative complications than previously reported (14,15,17,19). Clearly, CRP is not diagnostic in isolation and should be interpreted in the context of the patient’s overall clinical picture. The determined CRP cut-offs could be incorporated into management protocols to prompt consideration of clinical decisions such as further imaging or other investigations.
In some other series, CRP has been found to have poorer diagnostic accuracy compared to our findings. Späth et al. performed a similar study but expanded their cohort to include all upper gastrointestinal surgeries (28). One hypothesis to explain this difference is that their results were skewed by including multiple surgery types including patients undergoing hepatectomy. These patients, in particular, have poor hepatic reserve, and blood tests dependant on liver function may not accurately reflect a developing complication (29). The expected increase in CRP also varies between the surgical approach (i.e., open vs. laparoscopic) (30). Patient-specific factors such as comorbidities, ethnicity, lifestyle variables and genetics can also alter CRP concentrations in the body (31,32). Treating teams should be cognisant of inter-individual variabilities when interpreting and applying results.
The ΔCRP did not demonstrate statistically significant higher diagnostic accuracy than daily CRP for predicting SI complications. Although useful results were noted between POD 2 and POD 3, there were no significant results on any other day. CRP naturally rises early in the post-operative period because of the trauma associated with the surgery. Overall, we did not find that the daily change in CRP yielded clinically more useful results.
CRP exhibited a poor capacity to predict unplanned ICU admission. Admission to ICU can occur for a multitude of reasons other than complications that typically induce a CRP increase. Life-threatening complications such as arrythmias may warrant ICU admission but may not result in a significant CRP rise. Furthermore, clinicians may have a lower threshold for admitting patients with comorbid disease which may not be reflected in the CRP value. CRP also proved to be poor at predicting readmission to hospital within 30 days with only POD 2 demonstrating significant results. This was expected given the relatively short 19 hours half-life of CRP (33). Furthermore, patients may re-present to hospital for a multitude of reasons other than complications that typically induce a CRP rise (34).
There are several limitations that need to be considered when interpreting these results. Our research does not consider multiple complications occurring in the same patient. This is less relevant when using CRP to plan for early discharge but may limit the applicability of this research to patients who have more complex inpatient stays. Similarly, patient-specific factors such as bloods transfusions, intra-operative complications and extent of lymphadenectomy would likely influence post-operative CRP. Our research opted to include all patients who had a partial, total, or extended gastrectomy. Given the different nature of these procedures, our methods fail to determine the diagnostic accuracy of CRP for each operation in isolation. Furthermore, our study is a single institution analysis and may not be generalisable to other health care services. Factors such as patient demographics, comorbid disease and surgeon experience may skew these results. Accordingly, health services would benefit from developing their cut-offs which capture their unique health system profile. Potential biases may occur due to the retrospective analysis; however, the data is collected and maintained prospectively. Prospective studies are needed to determine if clinically applying cut-offs would result in earlier detection and reduced morbidity and mortality for patients and facilitate safer early discharge. Our study demonstrates CRP has a high negative predictive capacity early in the post-operative course and this is likely to be a clinically useful parameter.
Conclusions
CRP is a useful early test in predicting the absence of SI complications following gastrectomy. Our research suggests CRP can be predictive as early as POD 2 for this patient cohort. Patients who have a CRP below a pre-determine threshold (cut-off) can increase confidence in early discharge in the context of the whole clinical picture. Health services may benefit by determining similar cut-offs based on their own patient populations.
Acknowledgments
Dr. Matthew Read is supported by the Senior Lecturer Fellowship from the Royal Australasian College of Surgeons.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-675/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-675/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-675/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients were consented for data collection and subsequent analysis. The study was reviewed and approved by the St Vincent’s Hospital Melbourne Human Research Ethics Committee (HREC), which is registered with the National Health and Medical Research Council (No. HREC/16/SVHM/127).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lepage C, Sant M, Verdecchia A, et al. Operative mortality after gastric cancer resection and long-term survival differences across Europe. Br J Surg 2010;97:235-9. [Crossref] [PubMed]
- Meneveau M, Mehaffey JH, Adams PD, et al. Modifiable Factors to Prevent Prolonged Length of Stay after Sleeve Gastrectomy. Obes Surg 2019;29:1751-5. [Crossref] [PubMed]
- Jínek T, Adamčík L, Vrba R, et al. Risk factors and post-operative complications after gastrectomy for cancer. Rozhl Chir 2018;97:384-93.
- Yuan P, Wu Z, Li Z, et al. Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer 2019;19:833. [Crossref] [PubMed]
- Li Z, Bai B, Zhao Y, et al. Severity of complications and long-term survival after laparoscopic total gastrectomy with D2 lymph node dissection for advanced gastric cancer: A propensity score-matched, case-control study. Int J Surg 2018;54:62-9. [Crossref] [PubMed]
- Sivakumar J, Alnimri F, Johnson MA, et al. Health economic analysis of curative-intent gastrectomy for gastric carcinoma and the costs related to post-operative complications. ANZ J Surg 2021;91:E1-6. [Crossref] [PubMed]
- Hassan M, Tuckman HP, Patrick RH, et al. Hospital length of stay and probability of acquiring infection. Int J Pharm Healthc Mark 2010;4:324-38.
- Fox GJ, Trauer JM, McBryde E. Modelling the impact of COVID-19 on intensive care services in New South Wales. Med J Aust 2020;212:468-9. [Crossref] [PubMed]
- Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol 2001;38:189-97. [Crossref] [PubMed]
- Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol 2018;9:754. [Crossref] [PubMed]
- Gewurz H, Mold C, Siegel J, et al. C-reactive protein and the acute phase response. Adv Intern Med 1982;27:345-72.
- Komolafe O, Pereira SP, Davidson BR, et al. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database Syst Rev 2017;4:CD012645. [Crossref] [PubMed]
- Csendes JA, Muñoz ChA, Burgos L. Blood count and C-reactive protein evolution in gastric cancer patients with total gastrectomy surgery. Arq Bras Cir Dig 2014;27:234-6. [Crossref] [PubMed]
- Zhang K, Xi H, Wu X, et al. Ability of Serum C-Reactive Protein Concentrations to Predict Complications After Laparoscopy-Assisted Gastrectomy: A Prospective Cohort Study. Medicine (Baltimore) 2016;95:e3798. [Crossref] [PubMed]
- Shi J, Wu Z, Wang Q, et al. Clinical predictive efficacy of C-reactive protein for diagnosing infectious complications after gastric surgery. Therap Adv Gastroenterol 2020;13:1756284820936542. [Crossref] [PubMed]
- Obama K, Okabe H, Tsunoda S, et al. Clinical Significance of C-reactive Protein Level After Laparoscopic Gastrectomy: From a Viewpoint of Intra-Abdominal Complications. Int Surg 2015;100:1332-39.
- Kim EY, Yim HW, Park CH, et al. C-reactive protein can be an early predictor of postoperative complications after gastrectomy for gastric cancer. Surg Endosc 2017;31:445-54. Erratum in: Surg Endosc 2017;31:455. [Crossref] [PubMed]
- Warschkow R, Tarantino I, Ukegjini K, et al. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after gastroesophageal cancer surgery. Langenbecks Arch Surg 2012;397:727-36. [Crossref] [PubMed]
- Shishido Y, Fujitani K, Yamamoto K, et al. C-reactive protein on postoperative day 3 as a predictor of infectious complications following gastric cancer resection. Gastric Cancer 2016;19:293-301. [Crossref] [PubMed]
- van Winsen M, McSorley ST, McLeod R, et al. Postoperative C-reactive protein concentrations to predict infective complications following gastrectomy for cancer. J Surg Oncol 2021;124:1060-9. [Crossref] [PubMed]
- Okubo K, Arigami T, Matsushita D, et al. Clinical impact of creatine phosphokinase and c-reactive protein as predictors of postgastrectomy complications in patients with gastric cancer. BMC Cancer 2021;21:95. [Crossref] [PubMed]
- Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [Crossref] [PubMed]
- Harris PA, Taylor R, Minor BLThe REDCap consortium, et al. Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [Crossref] [PubMed]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007;115:654-7. [Crossref] [PubMed]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Späth C, Srinivasa S, Walsh M, et al. Role of post-operative serum C-reactive protein levels as a predictor of complications in upper gastrointestinal surgery. ANZ J Surg 2019;89:74-8. [Crossref] [PubMed]
- Rahman SH, Evans J, Toogood GJ, et al. Prognostic utility of postoperative C-reactive protein for posthepatectomy liver failure. Arch Surg 2008;143:247-53; discussion 253. [Crossref] [PubMed]
- Okholm C, Goetze JP, Svendsen LB, et al. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol 2014;49:1027-34. [Crossref] [PubMed]
- MacGregor AJ, Gallimore JR, Spector TD, et al. Genetic effects on baseline values of C-reactive protein and serum amyloid a protein: a comparison of monozygotic and dizygotic twins. Clin Chem 2004;50:130-4. [Crossref] [PubMed]
- Ford ES, Giles WH, Mokdad AH, et al. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem 2004;50:574-81. [Crossref] [PubMed]
- Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest 1993;91:1351-7. [Crossref] [PubMed]
- Jeong O, Kyu Park Y, Ran Jung M, et al. Analysis of 30-day postdischarge morbidity and readmission after radical gastrectomy for gastric carcinoma: a single-center study of 2107 patients with prospective data. Medicine (Baltimore) 2015;94:e259. [Crossref] [PubMed]


