
Neoadjuvant versus adjuvant imatinib in primary localized gastrointestinal stromal tumor
Highlight box
Key findings
• The effect of neoadjuvant therapy (NAT) with imatinib versus upfront resection (UR) on the outcomes of gastrointestinal stromal tumors (GIST) is unknown. Our earlier study showed that imatinib NAT reduced tumor size, metastasis, and tumor-related mortality in localized rectal GIST.
What is known and what is new?
• This larger retrospective study investigates the influence of NAT on prognostic outcomes in GISTs at all sites. Once again, we found that NAT decreases the risk of metastasis when compared to UR, particularly in non-gastric GIST.
What is the implication, and what should change now?
• These findings emphasize the importance of NAT in the integrated treatment of localized GIST. Further prospective validation is needed.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor in the gastrointestinal system. Most studies reported an annual incidence of 10–15 cases per million people (1). The majority of GISTs originate in the stomach (60–70%) and small bowel (20–25%), while colon, rectum, and esophagus (6.7%) are less common sites of origin (1). Most GISTs are caused by gain-of-function mutations of KIT and PDGFRA, which encode the receptor tyrosine kinase (RTK) (2). The emergence of the RTK inhibitor imatinib has dramatically enhanced the treatment of GIST (3). The foundation of curative treatment for primary localized GIST remains surgery (4). Imatinib-based adjuvant therapy (AT) improved survival results in intermediate—and high-risk patients compared with those of patients undergoing surgery alone (5).
Neoadjuvant therapy with imatinib (NAT) is one of the useful options for the multidisciplinary treatment of localized GISTs, especially for those in complex anatomical regions. Several retrospective studies and single-arm prospective studies indicated that neoadjuvant imatinib allowed a higher R0 resection rate and better oncologic outcomes compared with surgery alone, without compromising surgical safety (6-10). Consequently, the Chinese Society of Clinical Oncology (CSCO), the National Comprehensive Cancer Network (NCCN) in the United States, and the European Society for Medical Oncology (ESMO) have recommended that NAT be considered when R0 surgery is not feasible or implies major sequelae (11-13). However, the impact of NAT on the oncologic outcomes of this disease compared to upfront resection followed by adjuvant imatinib is uncertain in the absence of randomized control trial (RCT) data. RCTs are the gold standard by which we can determine the efficacy of treatments, but they are not always feasible or ethical (14). Compared to upfront resection (UR) paired with adjuvant therapy (AT) in localized rectal GIST, our earlier study demonstrated that NAT not only reduced tumor size but also decreased the probability of metastasis and tumor-related mortality (15).
This retrospective analysis aimed to investigate the additional effect of NAT in localized GISTs. We compared the oncologic outcomes of patients who got NAT followed by resection and AT to those of patients who received UR followed by AT. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-931/rc).
Methods
Study design and patients
This study was a retrospective observational study. A search for “GIST” was conducted in the prospectively archived pathologic database of the Sixth Affiliated of the Sun Yat-sen University between July 2007 and August 2021. The enrollment criteria were as follows: (I) complete clinical information and follow-up; (II) primary localized GIST and pathological diagnosis; and (III) patients undergoing surgical resection of primary lesions. The exclusion criteria were as follows: (I) patients with an initial diagnosis of metastatic disease; or (II) patients with no perioperative administration of imatinib. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (No. E2021104), and individual consent for this retrospective analysis was waived.
Exposure and outcomes
The patients with localized primary GIST were classified into two groups based on their treatment patterns (Groups A and B). Group A included patients who had received UR and AT for the prescribed period of 1 or 3 years if the tumor characteristics fulfilled the modified National Institutes of Health (NIH) consensus criteria for intermediate or high risk (12,16). Group B consisted of patients who had received NAT, surgical resection, and AT with the administration of perioperative imatinib for a recommended duration of 3 years (12). The choice of taking neoadjuvant therapy was based on the joint decision of the surgeon and patient, with the concern of finding a balance between R0 resection and function preservation. The outcomes of interest were distance recurrence-free survival (DRFS), local recurrence-free survival (LRFS), and overall survival (OS). LRFS was calculated from the date of surgery untill local tumor recurrence. DRFS was calculated from the date of surgery untill distant tumor recurrence. OS was measured from the date of the first diagnosis to the date of the patient’s death from any cause.
Data collection
The patient’s characteristics, clinicopathological features, treatment history, and survival data were extracted from the medical records. GIST was diagnosed on the basis of the histology and the immunohistochemical expression of KIT and/or DOG-1. According to the Response Evaluation Criteria in Solid Tumors 1.1 criteria (17), every 3–6 months a physical examination and chest, abdomen, and pelvic computed tomography or magnetic resonance imaging were conducted to assess the tumor’s response to NAT. Postoperative recurrence risk was classified as per modified NIH criteria (16). Before administering imatinib, it was proposed that each patient have a mutation analysis. DNA sequencing was carried out for the mutational analyses on KIT exons 9, 11, 13, and 17 as well as PDGFRA exons 12 and 18 using Sanger sequencing. The results were most recently updated in December 2021.
Statistical analysis
For continuous variables, descriptive statistics were expressed as the median and interquartile range (IQR), and for categorical variables, as numbers and percentages. The continuous variables (size and mitotic index) were converted into categorical variables, with category boundaries mirroring those used within the modified NIH consensus criteria (16). The Mann-Whitney or Fisher’s test was used to compare the distribution of baseline variables between groups. The survival curves were generated by the Kaplan-Meier method, and the log-rank tests were used to compare DRFS, LRFS, and OS between groups. Variables with clinical relevance and those heading toward significance (P<0.10) in univariate analysis were included in a multivariate Cox proportional-hazards model to investigate the independent effect of NAT administration on DRFS, LRFS, and OS. The propensity score adjustment using inverse probability of treatment weighting (IPTW) (18) was used to conduct a sensitivity analysis to confirm the robustness of the main result of COX regression. The survival analyses were then performed with the inverse probability of treatment weighting model (18).
Subgroup analyses were carried out to determine the benefits of neoadjuvant treatment across different patient cohorts, and interactions were tested. The statistical analysis was performed with SPSS (IBM SPSS 26.0; SPSS Inc.) and R software version 3.4.2 (Vienna, Austria). All tests were two-sided, and a P value of <0.05 indicated that the difference was statistically significant. For data analysis, the R packages IPW survival, survey, and boot were used (Appendix 1).
Results
Demographics and tumor features
A total of 922 patients with GIST were identified (Figure 1, Figure S1, and Table S1). Two hundred and eleven patients who met the selection criteria were included in the study. The median age was 58 years, and 59.2% were men. The most common primary tumor sites were the stomach (39.3%), followed by the small intestine (33.2%), rectum (23.7%), colon (0.9%), others (1.9%), and other unspecified sites (0.9%). A total of 140 patients (66.4%) underwent UR + AT (Group A), and 71 (33.6%) underwent NAT + resection + AT (Group B). The patient demographics, tumor characteristics, treatment, and pathological variables are summarized in Table 1. Based on the modified NIH consensus criteria (16), tumor size was categorized into the following 3 groups: ≤5 cm, 5–10 cm and >10 cm. Except for adjuvant imatinib (P=0.005), no significant differences were found between the two groups in the majority of parameters.
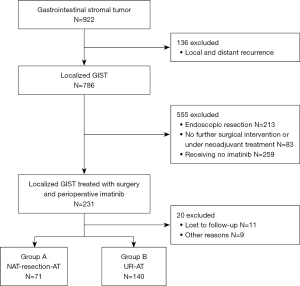
Table 1
| Variable | Category | Overall study population, n=211 (n, %) | Group A UR+AT, n=140 (n, %) | Group B NAT+R+AT, n=71 (n, %) | P valuea |
|---|---|---|---|---|---|
| Age, year | Median (IQR) | 57.5 (16.7) | 58.7 (17.6) | 54.8 (14.2) | 0.102 |
| Range | 24.8–81.4 | 24.8–81.4 | 26.9–72.8 | ||
| Gender | Male | 125 (59.2) | 82 (58.6) | 43 (60.6) | 0.781 |
| BMI | Median (IQR) | 22.9 (4.0) | 22.9 (4.5) | 23.0 (3.2) | 0.917 |
| Symptomatic | Yes, % | 169 (80.9) | 116 (84.1) | 53 (74.6) | 0.146 |
| Location | Non-gastric | 128 (60.7) | 79 (56.4) | 49 (69.0) | 0.077 |
| Gastric | 83 (39.3) | 61 (43.6) | 22 (31.0) | ||
| CD117b | Positive | 202 (95.7) | 136 (97.1) | 66 (93.0) | 0.289 |
| Weakly positive/negative | 9 (4.3) | 4 (2.9) | 5 (7.0) | ||
| DOG-1b | Positive | 200 (94.8) | 131 (93.6) | 69 (97.2) | 0.431 |
| Weakly positive/negative | 11 (5.2) | 9 (6.4) | 2 (2.8) | ||
| Molecular typing | KIT 11 | 75 (42.6) | 43 (42.2) | 32 (43.2) | 0.263 |
| KIT 9 | 10 (5.7) | 8 (7.8) | 2 (2.7) | ||
| KIT 13 | 1 (0.6) | 0 (0) | 1 (1.4) | ||
| KIT 17 | 2 (1.1) | 1 (1.0) | 1 (1.4) | ||
| PDGFRA 18 D842Vc | 1 (0.6) | 1 (1.0) | 0 (0) | ||
| PDGFRA 18 Non D842V | 2 (1.1) | 1 (1.0) | 1 (1.4) | ||
| Wild-type SDHB-deficient | 1 (0.6) | 1 (1.0) | 0 (0) | ||
| Missing | 84 (47.7) | 47 (46.1) | 37 (50.0) | ||
| Size, cm | ≤5 | 60 (28.4) | 45 (32.1) | 15 (21.1) | 0.125 |
| 5–10 | 108 (51.2) | 71 (50.7) | 37 (52.1) | ||
| >10 | 43 (20.4) | 24 (17.1) | 19 (26.8) | ||
| Rupture | Yes | 4 (1.9) | 3 (2.1) | 1 (1.4) | 0.712 |
| No | 207 (98.1) | 137 (97.9) | 70 (98.6) | ||
| Risk stratificationd | High risk | 133 (63.0) | 84 (60.0) | 49 (69.0) | 0.226 |
| Non high risk or unknown | 78 (37.0) | 56 (40.0) | 22 (31.0) | ||
| AT, month | Yes | 199 (94.3) | 137 (97.9) | 62 (87.3) | 0.005* |
| No | 12 (5.7) | 3 (2.1)e | 9 (12.7) | ||
| Median (IQR) | 17.0 (29.6) | 21.3 (24.2) | 12.0 (23.4) | <0.001* |
a, Statistical comparisons between Group A and Group B cases were performed with a chi-square test for categorical, with a t-test for numerical. b, Based on tissue specimens obtained before taking imatinib. c, The adjuvant imatinib was administrated for the patient with intermediate risk, in parallel with genotyping. After 4 weeks, imatinib was stopped as soon as a PDGFRA mutation in exon 18 (p.D842V) was detected. d, Risk stratification based on modified NIH consensus criteria. e, Taking imatinib no more than 7 days. *, Denotes statistically significant. NAT, neoadjuvant therapy with imatinib; R, resection; AT, adjuvant therapy with imatinib; UR, upfront resection; BMI, body mass index; IQR, interquartile range.
The risk stratification was based on the modified NIH consensus criteria (16). The grading by mitotic count was not accurate in regular biopsy (19), and NAT had evidence of a pathologic treatment effect that did not yield accurate mitotic information (20). Consequently, in the absence of mitotic count, the NAT group (Group B) consisted of 49 patients (69.0%) with high-risk tumors only based on tumor size and location (21 tumors of size >10 cm, 1 tumor of size >10 cm and rupture, and 27 tumors of size >5 cm with nongastic origin) (Tables S2,S3). In the UR group (Group A), the tumors were classified as high risk in 84 (60%) patients based on size, location, and mitotic count. The size and proportion of high-risk tumors did not differ significantly between groups A and B.
Treatments
NAT was given to 71 patients (33.6%), with a median duration of 5.6 months (range, 0.8–29.6 months). Sixty-five patients were evaluable for response. The disease control rate was 96.9%, the partial response rate (PR) was 44.6%, and the stable disease rate was 52.3% (Figure S2). One (1.5%) of the patients with PR disease (66% of the maximum change from baseline) after 13 months of NAT had achieved pathological complete response (pCR) postoperatively. In the entire cohort of 211 patients, all patients underwent surgical resection of the primary lesions.
AT was given to 137 (97.9%) patients in Group A and 62 (87.3%) patients in Group B (P=0.005) (Table 1), with the median duration of 21.3 months (range, 1.0–119 months) and 12.0 months (range, 1.0–63.9 months) (P<0.001), respectively. The percentage of patients who discontinued AT due to local or distant recurrence in groups A and B was 4.3% and 2.9%, respectively (P=0.143). In addition, one (1.4%) patient in Group B discontinued AT due to accidental death from a heart attack (Table S4). Sixteen patients (11.4%) in Group A and one (1.4%) in Group B had distant recurrence after AT discontinuation (P=0.043, Table S5).
Survival estimates
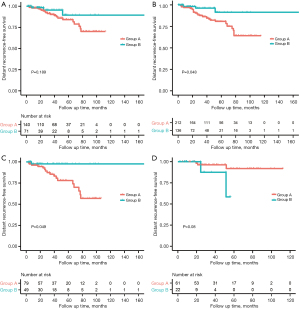
In the entire cohort (n=211), the median DRFS, LRFS, and OS were not reached, with a median follow-up time of 40.8 months (range, 4.5–147.1 months). At the last follow-up, 9 (4.3%) patients had died (Table 2). A total of 31 patients experienced recurrence, including 16 with distant recurrence, 8 with local recurrence, and 7 with local and distant co-recurrences (Table 2 and Tables S6,S7). The estimated 5-year DRFS, LRFS, and OS were 85.6%, 90.7%, and 92.5%, respectively. A log-rank test was used to compare Kaplan-Meier estimates between the groups. The 5-year DRFS, LRFS, and OS of groups A and B were 84.1% vs. 89.5% (P=0.189), 90.3% vs. 92.5% (P=0.42), and 93.80% vs. 87.50% (P=0.783) (Table 2 and Figure 2A,2B, Figure S3A,S3B).
Table 2
| Outcomes | Overall study population, n=211 | Group A: UR+AT, n=140 | Group B: NAT+R+AT, n=71 | P value |
|---|---|---|---|---|
| LRFS-event, n (%) | 15 (7.1) | 10 (7.1) | 5 (7.0) | 0.979 |
| DRFS-event, n (%) | 23 (10.9) | 20 (14.3) | 3 (4.2) | 0.037 |
| OS-event, n (%) | 9 (4.3) | 6 (4.3) | 3 (4.2) | 0.984 |
| 5-year LRFS | 90.70% | 90.30% | 92.50% | 0.42 |
| 5-year DRFS | 85.60% | 84.10% | 89.50% | 0.189 |
| 5-year OS | 92.50% | 93.80% | 87.50% | 0.783 |
NAT, neoadjuvant therapy with imatinib; R, resection; AT, adjuvant therapy with imatinib; UR, upfront resection; LRFS, local recurrence-free survival; DRFS, distance recurrence-free survival; OS, overall survival.
Univariate Cox regression analysis
Univariate Cox regression analyses were performed on factors predicting the DRFS, LRFS, and OS. Size, location, mitotic rate, and rupture predict independently the recurrence of primary GIST after resection (21). As mentioned earlier, the mitotic indexes were inadequate in the NAT group. Also, only four patientss of intraoperative rupture were confirmed. As a result, neither the mitotic index nor the rupture were included in the univariate or multivariate survival analysis. Adjuvant imatinib was associated with improved recurrence-free survival in patients with operable GIST (5). In our recent study, neoadjuvant imatinib was found to decrease the risk of metastasis and tumor-related deaths in patients with rectal GIST (15). Consequently, the tumor size, location, and neoadjuvant and adjuvant treatments were included in the univariate and multivariate survival analyses. Table 3 shows that tumor size was a predictor for all outcomes in the univariate analysis.
Table 3
| Variables | Category | DRFS | LRFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P-value | HR | 95% CI | P value | ||||
| NAT | Yes | 1.989 | (0.588–6.724) | 0.268 | 1.773 | (0.596–5.272) | 0.303 | 1.253 | (0.251–6.245) | 0.783 | ||
| Location | Gastric | 0.412 | (0.153–1.109) | 0.079 | 0.536 | (0.171–1.682) | 0.285 | 0.55 | (0.11–2.75) | 0.467 | ||
| Non-gastric | 1 | 1 | 1 | |||||||||
| Size, cm | ≤5 | 0.258 | (0.086–0.778) | 0.016* | 0.192 | (0.037–1.001) | 0.05 | 0.138 | (0.015–1.233) | 0.076 | ||
| 6–10 | 0.328 | (0.13–0.83) | 0.019* | 0.536 | (0.175–1.645) | 0.276 | 0.278 | (0.062–1.243) | 0.094 | |||
| >10 | 1 | 1 | 1 | |||||||||
| AT | Yes | 0.435 | (0.129–1.47) | 0.18 | 1.061 | (0.139–8.085) | 0.954 | 0.553 | (0.068–4.51) | 0.58 | ||
*, Denotes statistically significant. DRFS, distant recurrence-free survival; LRFS, local recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval. NAT, neoadjuvant therapy; AT, adjuvant therapy.
Multivariate Cox regression analysis
In the multivariate analysis, neoadjuvant treatment (HR =0.23, 95% CI: 0.056–0.96, P=0.044), tumor size ≤5 cm (P=0.014), and adjuvant imatinib (P=0.046) were associated with better DRFS, while tumor size ≤5 cm was associated with better LRFS (P=0.072) and OS (P=0.078) with marginal significance (Table 4). Notably, an interaction effect was observed between neoadjuvant treatment and tumor location on DRFS estimated from the survival data (interaction test P=0.006) (Table S8). In the subgroup analyses, NAT was associated with better DRFS in patients with non-gastric GISTs (HR =0.131, 95% CI: 0.017–0.989, P=0.049) (Table S4), while DRFS for patients with NAT did not significantly differ from that for patients with UR in patients with gastric GISTs (P=0.08) (Table S9). The Kaplan-Meier curves for DRFS of patients in Group A versus Group B stratified by location subgroups are shown in Figure 2C,2D. In patients with non-gastric GISTs, the estimated 5-year DRFS was 78.6% in Group A versus 97.3% in Group B (P=0.020).
Table 4
| Variables | Category | DRFS | LRFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| NAT | Yes | 0.232 | (0.056–0.96) | 0.044* | 1.476 | (0.477–4.569) | 0.5 | 0.955 | (0.171–5.345) | 0.958 | ||
| Location | Gastric | 0.48 | (0.173–1.332) | 0.159 | 0.638 | (0.2–2.035) | 0.447 | 0.669 | (0.129–3.461) | 0.632 | ||
| Non-gastric | 1 | |||||||||||
| Size, cm | ≤5 | 0.237 | (0.075–0.746) | 0.014* | 0.216 | (0.041–1.146) | 0.072 | 0.136 | (0.015–1.247) | 0.078 | ||
| 6–10 | 0.396 | (0.153–1.024) | 0.056 | 0.564 | (0.18–1.769) | 0.326 | 0.316 | (0.067–1.48) | 0.144 | |||
| >10 | 1 | 1 | 1 | |||||||||
| AT | Yes | 0.216 | (0.048–0.97) | 0.046* | 1.096 | (0.128–9.378) | 0.933 | 0.513 | (0.05–5.221) | 0.573 | ||
*, Denotes statistically significant. DRFS, distant recurrence-free survival; LRFS, local recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Sensitivity analyses using inverse treatment probability weighting and stratified analyses
We fitted a logistic model to obtain IPTW for our sensitivity analyses. After adjusting for tumor size, location, and AT, NAT-treated patients demonstrated a better DRFS than UR-treated ones (HR =0.26, 95% CI: 0.076–0.905, P=0.048; Table S10, Figure 2B). However, no significant relationship was discovered between treatment groups and LRFS or OS (HR =1.02, 95% CI: 0.314–3.34, P=0.969; Table S10 and Figure S3). The results of the IPTW analyses were similar to the original findings.
Discussion
The effect of NAT versus UR followed by AT on the oncologic outcomes of primary localized GIST is unknown. In this 14-year, single-center observational study, the oncologic endpoints of patients who got NAT followed by resection and AT were compared to those of patients who received UR followed by AT. The multivariate analysis by the Cox proportional-hazards model and sensitivity analysis by IPTW revealed that NAT was associated with better DRFS (HR =0.232, 95% CI: 0.0166–0.806, P=0.022), especially in patients with non-gastric GISTs (HR =0.131, 95% CI: 0.017–0.989, P=0.049).
Prior studies showed that the advantages of neoadjuvant imatinib included adequate downstaging, organ preservation, and meaningful survival benefits compared with surgery alone (6,10,22,23). Only a few studies investigated the impact of NAT on the outcomes of patients who also received AT. Using the National Cancer Database (2004–2016) for comparative research, Marqueen et al. discovered that receiving 3 months of NAT for localized GIST was associated with a slight improvement in OS compared with upfront surgery (7). However, the difference in OS between those in the NAT + R + AT group and those in the UR + AT group was no longer significant. Marqueen pointed out that the insufficient coding of treatment details, such as the length of AT, hampered the analysis’s interpretability. In our previous study (15), patients with localized rectal GIST who received NAT exhibited superior DRFS and disease-specific survival compared to those who underwent UR. Also, the correlation of OS with treatment groups was not statistically significant (P=0.07). The present study, including GIST from all sites with careful clinical follow-up and detailed information, revealed that NAT was independently associated with better DRFS (HR =0.23, 95% CI: 0.0166–0.806, P=0.022). Despite a minor numerical advantage for Group A (upfront resection) in 5-year OS, the log rank test revealed no difference in OS between the two groups (see Table 2, Figure S3B). This could be due to the small number of events, which made the estimated probability of survival at a given interval less accurate (24). A prolongation of the follow-up time period may be required. Furthermore, a trend for OS in favor of NAT was shown in the multivariate analysis. Although the present study was not an RCT, the sample size was relatively large, and the DRFS benefit of NAT was significant after adjusting for important GIST risk factors, such as tumor size, location, and adjuvant treatment, in both the Cox and IPTW models. Mitotic rate was also identified as an important prognostic factor (12). The proportions of high-risk patients were similar in the two groups (Table 1) in the present study. As in other neoadjuvant studies (20), we did not obtain accurate mitotic information before the preoperative administration of imatinib in Group B. This might lead to an underestimation of the proportion of high-risk patients in Group B (NAT). Even without adjustment for the mitotic count, the results showed that NAT was more beneficial compared with UR+AT in terms of DRFS. Hence, the true effect of NAT on the prognosis of GIST might be close to that estimated in the present study. In other words, NAT combined with surgery and AT might decrease the risk of metastasis compared with UR and AT.
The interaction of NAT with the site of tumor origin is interesting, but not surprising. For non-gastric GISTs in the present study, NAT was associated with better DRFS (HR =0.131, 95% CI: 0.017–0.989, P=0.049). This finding was consistent with our previous study on rectal GIST (15). In the pre-imatinib era, non-gastric GISTs were associated with less favorable outcomes than gastric GIST (16,25). Our data showed that more patients with non-gastric GIST underwent NAT than those with gastric GIST (38.3% vs. 26.5%, P=0.077, Table 1), particularly those with GISTs in the esophagus, duodenum, and rectum (73.7%). A population-based study by Ulrich Guller, including more than 5000 patients, showed that patients with non-gastric GIST had outcomes similar to those of patients with gastric GIST (26). Previous analyses verified that NAT offered several potential advantages, including preventing tumor rupture during surgery (10), eliminating micrometastases (27,28). Perioperative imatinib could counteract the unfavorable impact of non-gastric origin on the prognosis of GIST. For gastric GIST, only five events were observed in our study. It was difficult to assess the treatment effect in this subset of patients. In addition, a trend in favor of Group A was observed in gastric GIST. A possible reason might be that gastric GIST in Group B was characterized by larger tumor sizes (Table S11) and more high-risk tumors (Table S12) than in Group A. The marked disparate distribution of tumor size and risk classification between two groups in gastric GIST is known as “confounding by indication.” Allan et al. described “confounding by indication” as a bias in the connection between a treatment and its intended outcome caused by the severity of the underlying condition and its impact on the treatment decision. (29). Our findings were unable to invalidate the association between NAT and DRFS in stomach GIST. The majority of proximal gastric GISTs are KIT-mutant tumors (30), which also require function preservation. NAT has a key role in the therapy of proximal gastric GIST and distal gastric GIST with sensitive mutations. In clinical practice, NAT dismissmal in gastric GIST may be misleading. Further exploration of the link between NAT and DRFS in gastric GIST is needed.
The optimal length of NAT is undetermined, and the NCCN guidelines recommend a treatment duration of >6 months (12). In the present study, the median length of neoadjuvant therapy was 5.6 months (range 0.8–29.6 months), with an ORR of 49.4% in the entire cohort. The ORR in a neoadjuvant setting ranged from 60% to 65.9% when the median duration exceeded 6 months (10,15,31). Insufficient NAT probably led to unsatisfactory tumor shrinkage (32). In the univariate analyses, the duration of NAT was not related to DRFS or LRFS, but tumor size after NAT had a negative correlation with both DRFS (P=0.007, HR, 1.038, 1.01–1.067) and LRFS (P=0.022, HR, 1.03, 1.004–1.056). Hence, these results implied that the optimal length of NAT could be the time to the tumor nadir. Also, an imaging review for the tumor should be performed every 2–3 months during NAT so as not to miss the best operating time.
The recommended length of NAT + AT in patients undergoing neoadjuvant treatment is 3 years (12). It has been verified that imatinib should be taken postoperatively for at least 3 years in patients who have a high-risk GIST (5,33,34). Patients with ruptured localized GIST may require adjuvant imatinib treatment for 5 years, or even lifelong, since they have an extremely high chance of recurrence (35). Nishida et al. suggested that the micrometastases were not eradicated but remained under control for many years through drug therapy (36). The preclinical data implied that imatinib induced cellular quiescence but not death (37). This hypothesis was supported by the observation that the rates of disease recurrence similarly increased in both the 1-year and 3-year groups within 6–12 months of discontinuing adjuvant imatinib (38). In the present study, more patients in group A experienced distant recurrence after AT discontinuation than those in Group B (P=0.043, Table S5), which further confirmed that NAT might decrease distant recurrence. Also, the result increased the possibility that neoadjuvant therapy might allow for a shorter duration of perioperative imatinib for those with very high-risk GIST.
The local recurrence rate in the present study was 7.1%. No statistically significant association between the NAT and local recurrence was observed. The independent prognostic factors in LRFS were tumor size at diagnosis (P=0.039, HR, 0.196, 0.042–0.919) and adjuvant imatinib (P=0.014, HR, 0.956, 0.922–0.991). This finding was consistent with our previous study on rectal GIST and the published findings (8,15,39). Although NAT led to tumor downsizing, LRFS relied more on R0/R1 resection without rupture and postoperative imatinib.
However, this study had several inherent limitations due to its retrospective design. Given that the data of patients who underwent the resection of localized GIST and received perioperative imatinib was collected from our hospital database, sex, age, tumor size, and location were distributed equally among groups A and B (Table 1). Although we corrected for the differences in baseline characteristics, unknown confounders remained, for example, an inadequate baseline pre-NAT mitotic index. In the absence of an accurate mitotic index in Group B, the proportion of high-risk patients was similar for the two groups (Table 1). The originally high mitotic index might be masked by neoadjuvant imatinib, resulting in an underestimation of the true proportion of high-risk individuals in the NAT group. Even without mitotic count, when we adjusted the NAT effects for other prognostic key covariates such as tumor size, location, and AT by Cox regression and IPTW, the results showed that neoadjuvant imatinib (NAT) might be more beneficial than classic, postsurgical AT in terms of DRFS. The gold standard to assess the effect of neoadjuvant treatment is an RCT. However, neoadjuvant therapy is sometimes necessary for GIST, particularly when it is located at the esophagogastric junction and in the duodenum and rectum, to achieve complete resection and avoid extensive organ disruption. For ethical reasons, the random assignment of participants is not permitted under this circumstance. Thus, the guideline recommendation for neoadjuvant treatment for GIST is based on phase II single-arm trials or retrospective series (1-6). Moreover, no pertinent surgical data are available to determine the impact of margin and rupture status on the outcome of neoadjuvant GIST therapy.
Conclusions
Overall, this retrospective exploratory study compared the oncologic outcomes of NAT + surgery + AT with those of UR + AT in localized GIST. A multivariate Cox proportional-hazards regression model and the IPTW method were used to minimize the imbalances in key clinical variables and to make a robust estimate of the benefit of NAT over UR + AT in localized GIST. The findings suggested that NAT decreased the risk of metastasis, especially in patients with non-gastric GISTs, compared with UR and AT. Further prospective studies are warranted to verify these preliminary findings.
Acknowledgments
We thank Huiming Wang, Hui Wang for the useful discussion. We acknowledge Medsci for language editing.
Funding: This study was supported by Natural Science Foundation of Guangdong Province, China (No. 2019A1515010071 and No. 2021A1515010568), and National Natural Science Foundation of China (No. 81974369 and No. 81970495).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-931/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-931/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-931/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-931/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (No. E2021104), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol 2018;36:136-43. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients With High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up. JAMA Oncol 2020;6:1241-6. [Crossref] [PubMed]
- Cavnar MJ, Wang L, Balachandran VP, et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann Surg Oncol 2017;24:3972-80. [Crossref] [PubMed]
- Marqueen KE, Moshier E, Buckstein M, et al. Neoadjuvant therapy for gastrointestinal stromal tumors: A propensity score-weighted analysis. Int J Cancer 2021;149:177-85. [Crossref] [PubMed]
- Stuart E, Banerjee S, de la Torre J, et al. Frequent rectal gastrointestinal stromal tumor recurrences in the imatinib era: Retrospective analysis of an International Patient Registry. J Surg Oncol 2019;120:715-21. [Crossref] [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [Crossref] [PubMed]
- Kurokawa Y, Yang HK, Cho H, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 2017;117:25-32. [Crossref] [PubMed]
- Ye YJ, Qin SK, Shen L, et al. Guidelines of Chinese Society of Clinical Oncology on Diagnosis and Treatment of Gastrointestinal Stromal Tumor (2020 version). People's Medical Publishing House; 2021.
- National Comprehensive Cancer Network (NCCN) Clinical Practice guidelines in Oncology: Gastrointestinal Stromal Tumors (GISTs) (Version 1.2022). Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf. Accessed 30, October 2020.
- Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022;33:20-33. [Crossref] [PubMed]
- Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med 2018;210:2-21. [Crossref] [PubMed]
- Ling JY, Ding MM, Yang ZF, et al. Comparison of outcomes between neoadjuvant imatinib and upfront surgery in patients with localized rectal GIST: An inverse probability of treatment weighting analysis. J Surg Oncol 2021;124:1442-50. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. [Crossref] [PubMed]
- Kobara H, Mori H, Rafiq K, et al. Analysis of the amount of tissue sample necessary for mitotic count and Ki-67 index in gastrointestinal stromal tumor sampling. Oncol Rep 2015;33:215-22. [Crossref] [PubMed]
- Iwatsuki M, Harada K, Iwagami S, et al. Neoadjuvant and adjuvant therapy for gastrointestinal stromal tumors. Ann Gastroenterol Surg 2019;3:43-9. [Crossref] [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [Crossref] [PubMed]
- Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 2013;20:586-92. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res 2010;1:274-8. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Guller U, Tarantino I, Cerny T, et al. Revisiting a dogma: similar survival of patients with small bowel and gastric GIST. A population-based propensity score SEER analysis. Gastric Cancer 2017;20:49-60. [Crossref] [PubMed]
- Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Kang YK, Yook JH, Park YK, et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol 2021;39:2903-13. [Crossref] [PubMed]
- Allan V, Ramagopalan SV, Mardekian J, et al. Propensity score matching and inverse probability of treatment weighting to address confounding by indication in comparative effectiveness research of oral anticoagulants. J Comp Eff Res 2020;9:603-14. [Crossref] [PubMed]
- Sharma AK, de la Torre J, IJzerman NS, et al. Location of Gastrointestinal Stromal Tumor (GIST) in the Stomach Predicts Tumor Mutation Profile and Drug Sensitivity. Clin Cancer Res 2021;27:5334-42. [Crossref] [PubMed]
- Blesius A, Cassier PA, Bertucci F, et al. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer 2011;11:72. [Crossref] [PubMed]
- Ramaswamy A, Jain D, Sahu A, et al. Neoadjuvant imatinib: longer the better, need to modify risk stratification for adjuvant imatinib. J Gastrointest Oncol 2016;7:624-31. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015;33:4276-83. [Crossref] [PubMed]
- Nishida T, Hølmebakk T, Raut CP, et al. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann Surg Oncol 2019;26:1669-75. [Crossref] [PubMed]
- Nishida T, Sato S, Ozaka M, et al. Long-term adjuvant therapy for high-risk gastrointestinal stromal tumors in the real world. Gastric Cancer 2022;25:956-65. [Crossref] [PubMed]
- Nik Nabil WN, Xi Z, Liu M, et al. Advances in therapeutic agents targeting quiescent cancer cells. Acta Materia Medica 2022;1:56-71.
- Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016;34:244-50. [Crossref] [PubMed]
- Yasui M, Tsujinaka T, Mori M, et al. Characteristics and prognosis of rectal gastrointestinal stromal tumors: an analysis of registry data. Surg Today 2017;47:1188-94. [Crossref] [PubMed]


