
Advanced lung cancer inflammation index predicts the outcomes of patients with non-metastatic gastric cancer after radical surgical resection
Introduction
Gastric cancer (GC) is the fifth most common malignancy worldwide, and its mortality is the third-highest among all malignant tumors worldwide (1). Radical resection is a fundamental method of achieving long-term survival for patients with non-metastatic GC. For patients with non-metastatic GC, preoperative prognostic and predictive biomarkers may help identify high-risk patients, predict the outcome of GC, and even offer a therapeutic strategy.
One generally accepted theory contends that the progression of the malignant tumor induces an impaired nutritional status (2). Several convenient indicators, including serum albumin (ALB), prealbumin (PA), and body mass index (BMI), reflect the nutritional status. Studies have shown that these markers are important for predicting the prognosis of various malignant tumors, including GC (3-11). In addition, the immune function and state of inflammation are also associated with malignant tumors. This correlation is reflected in the level of serological markers. In recent years, studies have shown that serum inflammatory markers, such as the neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), have prognostic value for predicting the outcomes of melanoma, GC, and breast cancer (12-14).
The advanced lung cancer inflammation index (ALI) was first developed as a valuable prognostic biomarker for non-small cell lung cancer (15). It integrates the nutritional markers BMI and ALB with the serum inflammation indicator NLR to assess the prognosis of malignant tumors. Several studies have confirmed that the ALI has significant value in the prognostic evaluation of patients with colorectal cancer, nasopharyngeal carcinoma, or diffuse large B cell lymphoma (16-18). However, the prognostic significance of the ALI in non-metastatic GC remains unclear.
GC may cause upper gastrointestinal symptoms, including nausea, vomiting, bloating, abdominal pain, etc. Therefore, nutritional impairment may be present in patients with non-metastatic GC (19). Additionally, GC induces systemic inflammation alteration. Hence, a thorough investigation of the predictive value of the ALI for the prognosis of non-metastatic GC is of great importance. In the present study, we investigated the prognostic values of the ALI in non-metastatic GC patients who underwent radical surgical resection and identified high-risk patients to guide adjuvant therapy after potentially curative surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-657/rc).
Methods
Study population
A total of 1,657 patients with stage I to III gastric adenocarcinoma who were treatment naïve and underwent surgery at the Sixth Affiliated Hospital of Sun Yat-sen University from January 2008 to September 2020 were included in the study cohort. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the Ethical Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (No. 2021ZSLYEC-325). The requirement for informed consent for this retrospective study was waived.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) all included patients were admitted to hospitals for primary diagnosis and were treatment-naïve; (II) patients were pathologically diagnosed with primary GC in Tumor Node Metastasis (TNM) stage I to III; (III) patients underwent radical surgical resection with lymph node dissection; and (IV) all clinical data for patients were available. Patients were excluded if they met any of the following criteria: (I) had non-detailed or incomplete clinical data and follow-up data; (II) had non-primary gastric tumors; (III) had a history of malignant tumors other than GC; (IV) had gastric stump carcinoma; (V) received palliative resections; (VI) had clinical evidence of infection, other inflammation such as inflammatory bowel diseases, haematology disease, or used haematology-influenced drugs within 1 month preoperatively; and (VII) had an acute myocardial infarction, pulmonary embolism, or cerebrovascular accident within 1 month preoperatively. A flowchart of the patient selection process is shown in Figure 1.
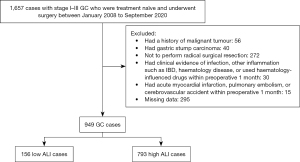
Data collection
All clinicopathological data were collected from the cancer database of the Sixth Affiliated Hospital of Sun Yat-sen University. The clinicopathological data included age, sex, weight, height, preoperative serum indices, and pathological features. Follow-up data were collected from the hospital’s follow-up office.
Preoperative serum indices included ALB, absolute neutrophil count, and absolute lymphocyte count. The weight, height, and preoperative serum indices were obtained within 2 weeks before patients underwent surgical resection for GC. BMI was calculated as weight divided by height squared. NLR was calculated as absolute neutrophil count divided by absolute lymphocyte count. According to the literature, the ALI was calculated as follows: ALI = BMI (kg/m2) × ALB (g/dL)/NLR.
The pathological features included histological type, histological grade, T stage, TNM stage classification, lymph node metastasis, vessel invasion, and perineural invasion. The histological type of GC was classified into differentiated adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma. Histological grades were categorized as well-differentiated, moderately differentiated, and poorly differentiated. TNM stage classification was determined according to the guidelines of the American Joint Committee on Cancer TNM staging system (8th edition) (20).
The primary oncologic outcomes were the overall survival (OS) and cancer-specific survival (CSS) rates. OS was defined as the period from the date of surgical resection of primary tumors to the date of death from any cause or until the last contact. CSS was defined as the period from the date of surgical resection of primary tumors to the date of death from GC.
Statistical analysis
Taking 5-year OS status and 5-year OS time as events, the optimal cut-off point of the ALI in this cohort was determined using the X-tile 3.6.1 software (Yale University, New Haven, Haven, CT, USA). Statistical evaluation was performed using the R software, version 4.1.2 (http://www.r-project.org). Continuous variables were tested for normal distribution using the Shapiro-Wilk normality test. Normally distributed continuous variables were expressed as the mean ± standard deviation and non-normally distributed continuous variables were expressed as the median (interquartile range). The Mann-Whitney U test or independent samples t-test was used for the analysis of continuous variables. Pearson’s chi-square test or Fisher’s exact test was used for the analysis of categorical variables. Bonferroni correction was applied for post hoc analysis after Chi-squared testing.
OS and CCS were computed as measures of patient survival. The Kaplan-Meier method was employed to estimate the survival rates, and the log-rank significance test was used to estimate the survival differences among various subgroups. The median follow-up duration was calculated by the reverse Kaplan-Meier method. Univariable and multivariable Cox regression analyses were performed to evaluate the prognostic factors in OS and CCS; variables with P values <0.05 in the univariable analysis were included in the multivariable Cox regression.
To reduce selection bias and potential confounding factors, propensity score matching (PSM) (1:3) was conducted using a nearest-neighbour algorithm to adjust for demographical and clinical covariates. High or low preoperative ALI was designated as the objective factor. With the application of logistic regression analysis, a continuous propensity score ranging from 0 to 1 was generated. The adjusted covariates in PSM included age, sex, T stage (T1/2 or T3/4), lymph node metastasis (presence or absence), lymphatic vessel invasion (presence or absence), and perineural invasion (presence or absence). The caliper was 0.10. PSM and survival analysis were conducted using the following R packages: “Matchit”, “survival”, and “survminer”. All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Clinicopathological characteristics of the included patients
A total of 949 patients were included in this analysis, including 615 men and 334 women, aged 20 to 97 years (median age, 60 years). The clinicopathologic characteristics of these patients are summarized in Table 1. In this cohort, 278 had stage I (29.29%), 306 had stage II (32.24%), and 365 had stage III disease (38.46%). The median BMI, ALB, and NLR were 21.97 kg/m2, 4.07 g/dL, and 1.94, respectively. The mean ALI score was 49.71±26.91, and the median value was 45.25 (range, 2.19–185.67).
Table 1
| Characteristics | Overall (N=949) | Low ALI (N=156) | High ALI (N=793) | P value |
|---|---|---|---|---|
| Age (years), n (%) | 0.004* | |||
| ≤60 | 493 (51.95) | 64 (41.03) | 429 (54.10) | |
| >60 | 456 (48.05) | 92 (58.97) | 364 (45.90) | |
| Sex, n (%) | 0.037* | |||
| Female | 334 (35.19) | 43 (27.56) | 291 (36.70) | |
| Male | 615 (64.81) | 113 (72.44) | 502 (63.30) | |
| T stage, n (%) | 0.001* | |||
| T1/2 | 358 (37.72) | 40 (25.64) | 318 (40.10) | |
| T3/4 | 591 (62.28) | 116 (74.36) | 475 (59.90) | |
| Lymph node metastasis, n (%) | 0.030* | |||
| Absence | 406 (42.78) | 54 (34.62) | 352 (44.39) | |
| Presence | 543 (57.22) | 102 (65.38) | 441 (55.61) | |
| TNM stage, n (%) | 0.004* | |||
| I | 278 (29.29) | 32 (20.51) | 246 (31.02) | |
| II | 306 (32.24) | 47 (30.13) | 259 (32.66) | |
| III | 365 (38.46) | 77 (49.36) | 288 (36.32) | |
| Histological type, n (%) | 0.970 | |||
| Differentiated adenocarcinoma | 799 (84.19) | 132 (84.62) | 667 (84.11) | |
| Signet ring cell or mucinous adenocarcinoma | 150 (15.81) | 24 (15.38) | 126 (15.89) | |
| Histological grade, n (%) | 0.144 | |||
| Well/moderately differentiated | 216 (22.76) | 43 (27.56) | 173 (21.82) | |
| Poorly differentiated | 733 (77.24) | 113 (72.44) | 620 (78.18) | |
| Vessel invasion, n (%) | 0.001* | |||
| Absence | 657 (69.23) | 90 (57.69) | 567 (71.50) | |
| Presence | 292 (30.77) | 66 (42.31) | 226 (28.50) | |
| Perineural invasion, n (%) | 0.052 | |||
| Absence | 562 (59.22) | 81 (51.92) | 481 (60.66) | |
| Presence | 387 (40.78) | 75 (48.08) | 312 (39.34) | <0.001* |
| ALI, mean (SD) | 49.71 (26.91) | 16.33 (6.27) | 56.28 (24.43) | <0.001* |
| NLR, mean (SD) | 2.57 (2.66) | 6.17 (5.04) | 1.87 (0.67) | <0.001* |
| ALB (g/dL), mean (SD) | 4.05 (0.48) | 3.73 (0.65) | 4.11 (0.41) | <0.001* |
| BMI (kg/m2), mean (SD) | 22.13 (3.21) | 20.82 (3.09) | 22.38 (3.17) | |
| Overall survival, n (%) | <0.001* | |||
| Alive | 759 (79.98) | 108 (69.23) | 651 (82.09) | |
| Dead | 190 (20.02) | 48 (30.77) | 142 (17.91) | |
| Cancer-specific survival, n (%) | <0.001* | |||
| Alive | 759 (79.98) | 108 (69.23) | 651 (82.09) | |
| Dead due to cancer | 165 (17.39) | 39 (25.00) | 126 (15.89) | |
| Dead of other cause | 25 (2.63) | 9 (5.77) | 16 (2.02) |
*, P value is statistically significant (P<0.05). ALI, advanced lung cancer inflammation index; NLR, neutrophil-lymphocyte ratio; ALB, Albumin; BMI, body mass index.
The median follow-up duration was 35 months. The 1-, 3-, and 5-year OS rates in this cohort were 92.9%, 78.0%, and 70.8%, respectively. The median OS was not reached among this patient population.
Correlation between the ALI and clinicopathologic factors
According to the X-tile software, the optimal cut-off value for the ALI for this cohort was 24.81. The 949 included patients then were divided into high and low ALI groups (793 vs. 156, respectively). The relationship between the ALI and clinicopathological factors is shown in Table 1. Numerous factors influence preoperative ALI. Lower preoperative ALI was significantly correlated with the well-established clinicopathologic factors of progressive disease in patients with GC, including male gender (P=0.037), older age (P=0.004), T3/4 stage (P=0.001), lymph node metastasis (P=0.030), TNM stage classification progression (P=0.004), and vessel invasion (P=0.001) (Table 1). There were no significant differences between the two ALI groups in terms of tumor-related factors, such as histological type, histological grade, and perineural invasion.
Survival analysis according to the ALI
We performed the Kaplan-Meier survival analysis to evaluate the prognostic impact of the ALI. Patients with low ALI had a significantly worse prognosis compared to those with high ALI in terms of OS (P<0.001, log-rank test; Figure 2A) and CSS (P=0.001, log-rank test; Figure 2B).

Univariable and multivariable analyses
Univariable and multivariable Cox regression analyses were performed to clarify the predictive potential of the ALI for the prognosis of non-metastatic GC. According to the univariable analysis, age, ALI, T stage, lymph node metastasis, perineural invasion, and vessel invasion were associated with worse prognosis in patients with non-metastatic GC (Table 2). In the multivariable analysis of variables from the univariable analysis with P<0.05, older age [hazard ratio (HR) =1.71; 95% confidence interval (CI), 1.27–2.29; P<0.001], low ALI (HR =1.55; 95% CI, 1.11–2.16; P=0.010), perineural invasion (HR =1.69; 95% CI, 1.23–2.33; P=0.001), T3/4 stage (HR =3.89; 95% CI, 2.21–6.86; P<0.001), lymph node metastasis (HR =2.43; 95% CI, 1.62–3.63; P<0.001), and vessel invasion (HR =1.37; 95% CI, 1.01–1.84; P=0.041) were independently associated with OS.
Table 2
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age >60 years | 1.60 (1.20–2.13) | 0.001 | 1.71 (1.27–2.29) | <0.001* | |
| Sex (male) | 1.01 (0.75–1.36) | 0.957 | – | – | |
| ALI score (low) | 1.97 (1.42–2.73) | <0.001 | 1.55 (1.11–2.16) | 0.010* | |
| T3/4 stage | 7.48 (4.41–12.67) | <0.001 | 3.89 (2.21–6.86) | <0.001* | |
| Lymph node metastasis (presence) | 4.18 (2.85–6.11) | <0.001 | 2.43 (1.62–3.63) | <0.001* | |
| Histological grade (poorly differentiated) | 1.44 (0.99–2.08) | 0.056 | – | – | |
| Histological type (signet ring cell or mucinous adenocarcinoma) | 1.07 (0.74–1.54) | 0.723 | – | – | |
| Perineural invasion (presence) | 3.03 (2.26–4.07) | <0.001 | 1.69 (1.23–2.33) | 0.001* | |
| Vessel invasion (presence) | 2.45 (1.84–3.26) | <0.001 | 1.37 (1.01–1.84) | 0.041* | |
*, P value is statistically significant (P<0.05). ALI, advanced lung cancer inflammation index; HR, hazard ratio; CI, confidence interval.
In addition, we also performed univariable and multivariable Cox regression analyses to determine the potential value of the ALI for CCS. Similar to the findings for OS, multivariable analyses for CCS also showed that low ALI was an independent prognostic factor (HR =1.46; 95% CI, 1.01–2.10; P=0.043) (Table 3).
Table 3
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age >60 years | 1.38 (1.02–1.88) | 0.039 | 1.56 (1.13–2.14) | 0.006* | |
| Sex (male) | 0.94 (0.68–1.30) | 0.717 | – | – | |
| ALI score (low) | 1.81 (1.27–2.60) | 0.001 | 1.46 (1.01–2.10) | 0.043* | |
| T3/4 stage | 9.92 (5.23–18.81) | <0.001 | 5.11 (2.60–10.06) | <0.001* | |
| Lymph node metastasis (presence) | 4.28 (2.83–6.46) | <0.001 | 2.33 (1.50–3.6) | <0.001* | |
| Histological grade (poorly differentiated) | 1.74 (1.13–2.66) | 0.011 | 1.41 (0.91–2.18) | 0.125 | |
| Histological type (signet ring cell or mucinous adenocarcinoma) | 1.16 (0.79–1.70) | 0.459 | – | – | |
| Perineural invasion (presence) | 3.27 (2.38–4.50) | <0.001 | 1.69 (1.20–2.37) | 0.003* | |
| Vessel invasion (presence) | 2.51 (1.85–3.41) | <0.001 | 1.36 (0.99–1.88) | 0.059 | |
*, P value is statistically significant (P<0.05). ALI, advanced lung cancer inflammation index; HR, hazard ratio; CI, confidence interval.
Propensity score matching analysis
Furthermore, PSM was performed to minimize selection bias and obtain credible results. Table 4 demonstrates the baseline patient characteristics after PSM analysis. Further univariable and multivariable Cox regression analyses were conducted. In the matched cohorts, no significant covariate difference was found between the two groups (P>0.05). A Kaplan-Meier survival curve demonstrated that GC patients with low ALI had worse OS (P=0.011, log-rank test; Figure 3A) and CSS (P=0.080, log-rank test; Figure 3B) than those with high ALI.
Table 4
| Characteristics | Overall (N=586) | Low ALI (N=156) | High ALI (N=430) | P value |
|---|---|---|---|---|
| Age (years), n (%) | 0.626 | |||
| ≤60 | 252 (43.00) | 64 (41.03) | 188 (43.72) | |
| >60 | 334 (57.00) | 92 (58.97) | 242 (56.28) | |
| Sex, n (%) | 0.886 | |||
| Female | 166 (28.33) | 43 (27.56) | 123 (28.60) | |
| Male | 420 (71.67) | 113 (72.44) | 307 (71.40) | |
| T stage, n (%) | 0.661 | |||
| T1/2 | 160 (27.30) | 40 (25.64) | 120 (27.91) | |
| T3/4 | 426 (72.70) | 116 (74.36) | 310 (72.09) | |
| Lymph node metastasis, n (%) | 1.000 | |||
| Absence | 202 (34.47) | 54 (34.62) | 148 (34.42) | |
| Presence | 384 (65.53) | 102 (65.38) | 282 (65.58) | |
| TNM stage, n (%) | 0.845 | |||
| I | 127 (21.67) | 32 (20.51) | 95 (22.09) | |
| II | 181 (30.89) | 47 (30.13) | 134 (31.16) | |
| III | 278 (47.44) | 77 (49.36) | 201 (46.74) | |
| Histological type, n (%) | 0.894 | |||
| Differentiated adenocarcinoma | 492 (83.96) | 132 (84.62) | 360 (83.72) | |
| Signet ring cell or mucinous adenocarcinoma | 94 (16.04) | 24 (15.38) | 70 (16.28) | |
| Histological grade, n (%) | 0.398 | |||
| Well/moderately differentiated | 145 (24.74) | 43 (27.56) | 102 (23.72) | |
| Poorly differentiated | 441 (75.26) | 113 (72.44) | 328 (76.28) | |
| Vessel invasion, n (%) | 0.610 | |||
| Absence | 350 (59.73) | 90 (57.69) | 260 (60.47) | |
| Presence | 236 (40.27) | 66 (42.31) | 170 (39.53) | |
| Perineural invasion, n (%) | 0.809 | |||
| Absence | 311 (53.07) | 81 (51.92) | 230 (53.49) | |
| Presence | 275 (46.93) | 75 (48.08) | 200 (46.51) | <0.001* |
| ALI, mean (SD) | 44.09 (26.10) | 16.33 (6.27) | 54.15 (23.10) | <0.001* |
| NLR, mean (SD) | 3.04 (3.26) | 6.17 (5.04) | 1.90 (0.67) | <0.001* |
| ALB (g/dL), mean (SD) | 4.00 (0.51) | 3.73 (0.65) | 4.08 (0.42) | <0.001* |
| BMI (kg/m2), mean (SD) | 21.81 (3.11) | 20.82 (3.09) | 22.175 (3.04) | |
| Overall survival, n (%) | 0.229 | |||
| Alive | 429 (73.21) | 108 (69.23) | 321 (74.65) | |
| Dead | 157 (26.79) | 48 (30.77) | 109 (25.35) | |
| Cancer-specific survival, n (%) | 0.089 | |||
| Alive | 429 (73.21) | 108 (69.23) | 321 (74.65) | |
| Dead due to cancer | 138 (23.55) | 39 (25.00) | 99 (23.02) | |
| Dead of other cause | 19 (3.24) | 9 (5.77) | 10 (2.33) |
*, P value is statistically significant (P<0.05). ALI, advanced lung cancer inflammation index; NLR, neutrophil-lymphocyte ratio; ALB, albumin; BMI, body mass index.

Multivariable Cox analysis indicated that low ALI was an independent prognostic factor for OS (HR =1.51; 95% CI, 1.08–2.13; P=0.017) (Table 5). However, univariable analyses showed that low ALI was not an independent prognostic factor for CSS (HR =1.39; 95% CI, 0.96–2.02; P=0.082) (Table 6). Overall, these results revealed that the ALI could serve as an independent factor for predicting the prognosis of patients with non-metastatic GC.
Table 5
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age >60 years | 1.52 (1.10–2.11) | 0.011 | 1.8 (1.28–2.53) | 0.001* | |
| Sex (male) | 0.82 (0.59–1.15) | 0.258 | – | – | |
| ALI score (low) | 1.55 (1.11–2.19) | 0.011 | 1.51 (1.08–2.13) | 0.017* | |
| T3/4 stage | 7.36 (3.88–13.99) | <0.001 | 3.53 (1.76–7.05) | <0.001* | |
| Lymph node metastasis (presence) | 3.57 (2.34–5.44) | <0.001 | 1.92 (1.22–3.02) | 0.005* | |
| Histological grade (poorly differentiated) | 1.87 (1.23–2.85) | 0.004 | 1.41 (0.91–2.18) | 0.124 | |
| Histological type (signet ring cell or mucinous adenocarcinoma) | 1.23 (0.82–1.83) | 0.312 | – | – | |
| Perineural invasion (presence) | 2.96 (2.13–4.13) | <0.001 | 1.77 (1.23–2.54) | 0.002* | |
| Vessel invasion (presence) | 2.39 (1.74–3.27) | <0.001 | 1.43 (1.02–1.99) | 0.036* | |
*, P value is statistically significant (P<0.05). ALI, advanced lung cancer inflammation index; HR, hazard ratio; CI, confidence interval.
Table 6
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age >60 years | 1.33 (0.95–1.88) | 0.100 | – | – | |
| Sex (male) | 0.72 (0.51–1.02) | 0.064 | – | – | |
| ALI score (low) | 1.39 (0.96–2.02) | 0.082 | – | – | |
| T3/4 stage | 9.36 (4.37–20.04) | <0.001 | 5.10 (2.27–11.48) | <0.001* | |
| Lymph node metastasis (presence) | 3.50 (2.23–5.48) | <0.001 | 1.82 (1.12–2.94) | 0.015* | |
| Histological grade (poorly differentiated) | 2.32 (1.43–3.77) | 0.001 | 1.55 (0.94–2.54) | 0.085 | |
| Histology type (signet ring cell or mucinous adenocarcinoma) | 1.28 (0.84–1.96) | 0.244 | – | – | |
| Perineural invasion (presence) | 3.02 (2.12–4.31) | <0.001 | 1.49 (1.03–2.18) | 0.036* | |
| Vessel invasion (presence) | 2.34 (1.67–3.27) | <0.001 | 1.34 (0.94–1.91) | 0.106 | |
*, P value is statistically significant (P<0.05). ALI, advanced lung cancer inflammation index; HR, hazard ratio; CI, confidence interval.
Discussion
In the present study, we assessed the relationships between the ALI and clinical features and investigated the potential predictive and prognostic value of ALI in non-metastatic GC patients who underwent radical surgical resection. We found ALI was associated with several clinical features, including sex, age, T stage, lymph node metastasis, TNM stage, and vessel invasion. The results also showed that low ALI was an independent predictor of poor outcomes in non-metastatic GC patients who underwent radical surgical resection. Finally, we performed PSM analyses to support the significant prognostic value of ALI.
Systemic inflammation status has been associated not only with tumor invasion and metastasis but also with the prognosis of malignant tumors. Circulatory inflammatory cells, which can be simply and directly checked by complete blood counts, reflect the systemic inflammatory status. Therefore, the link between inflammation and the prognosis of malignant tumors has been extensively studied. Studies have reported that several markers of systemic inflammation including NLR, PLR, and C-reactive protein have been shown to have prognostic value in patients with malignant tumors, such as breast cancer, non-small cell lung cancer, GC, pancreatic cancer, and colorectal cancer (14,21-25). NLR comprises both neutrophil and lymphocyte counts. According to the literature, NLR has prognostic value in various malignant tumors (14,21,25). The molecular mechanisms underlying NLR as a prognostic biomarker are still unknown but may represent a dynamic relationship between the NLR and the immune system (26). Neutrophils can release growth factors to promote angiogenesis, tumorigenesis, and metastasis. This immune cell is primarily responsible for the function of tumor immunity. Therefore, the NLR, which implies a relative change between neutrophils and lymphocytes, reflects a functional change between antitumor and protumour. Based on previous reports, the NLR is an outcome predictor in advanced and metastatic GC (22,27).
BMI is the most widely used measure of body fat and an objective index for systemic nutrition. Prior studies have demonstrated that BMI is an important prognostic biomarker for GC (4). A retrospective study that analyzed 7,765 patients with GC undergoing curative gastrectomy pointed out that patients who were overweight or mildly to moderately obese (BMI: 23–30 kg/m2) preoperatively had better OS and disease-specific survival compared with normal-weight cases (4). Parisi et al. reported that BMI was the major prognostic factor in advanced GC patients treated with second-line ramucirumab (28). Park et al. demonstrated that BMI was closely related to the prognosis of patients with stage II or III GC (19). According to epidemiological surveys, the mean BMI of the Chinese population is lower than that of the North American and Western European populations (29). The mean BMI of patients in the present study was 22.13 kg/m2, which is consistent with the above-mentioned survey results. Therefore, the optimal cut-off of the ALI in this study may apply to the Chinese population. ALB, which is synthesized by the liver, has been used as a marker for nutritional status in cancer patients. A low ALB level is associated with the prognosis of malignant tumors, including GC, non-small cell lung cancer, and colorectal cancer (3,10,30).
The ALI is calculated from the NLR, ALB, and BMI, thereby reflecting nutritional status and systemic inflammation. It was first reported by Jafri et al. who used it to evaluate the prognosis of lung cancer (15). ALI was then reported to have prognostic value for other malignant tumors, including colorectal cancer, nasopharyngeal carcinoma, and diffuse large B cell lymphoma (16-18). Specifically for GC, Yin et al. studied the association between the ALI and survival in GC patients with stage I to stage IV disease who had undergone gastrectomy (31). Shibutani et al. reported on the prognostic value of the ALI for patients with unresectable metastatic colorectal cancer (32). In addition, the ALI has been shown to have prognostic value in some locally advanced or metastatic cancer, including nasopharyngeal carcinoma and non-small cell lung cancer (17,33). These studies focused on advanced malignant tumors, particularly stage IV tumors. Compared with patients with metastatic tumors, those with non-metastatic tumors are more likely to have better performance status and less likely to have an impacted inflammatory status or nutritional status. Therefore, it is unclear whether the ALI has any predictive role for patients with non-metastatic GC.
Notably, as an upper digestive tract cancer, GC can easily induce alimentary symptoms during tumor development and progression, such as abdominal distension, pain, nausea, and vomiting. In addition, GC is an inflammatory-related tumor (34). Therefore, altered systemic inflammation and impaired nutritional status may have been present in patients with non-metastatic GC, which reflects the ALI change. In this study, we demonstrated that low ALI was an independent predictor of poor outcomes in non-metastatic GC patients who underwent radical surgery. The decreased ALI implies that patients with early-stage GC may require close follow-up, and patients with local advanced GC may require more aggressive adjuvant chemotherapy. Therefore, the ALI has prognostic value not only for metastatic GC patients but also for non-metastatic GC patients.
There are some potential limitations of this study that should be considered. Firstly, Firstly, although we assessed a large sample of patients with non-metastatic GC, this is a retrospective and single-centre study. Therefore, several forms of bias, such as selection bias or observer bias, may have been present in this study. Secondly, we could not obtain the dynamic of ALI at multiple time points because we only focus on ALI at a single time point and there were limited retrospective studies. Thirdly, the evaluation of systemic nutrition can be complicated. BMI and ALB were selected to evaluate systemic nutrition in this study, which might have some limitations with respect to specificity and sensitivity. To overcome these limitations, a multi-centre and prospective study is needed to validate these results.
Conclusions
In this study, we demonstrated that preoperative ALI was an independent prognostic marker in patients with non-metastatic GC who underwent radical surgery. The ALI is a simple, convenient, and low-cost prognostic indicator that can help identify high-risk cases, predict prognosis, and assist surgeons in optimizing clinical decisions.
Acknowledgments
We appreciated the cancer database of Sixth Affiliated Hospital of Sun Yat-sen University for helping us collect the data.
Funding: This study was supported by the National Natural Science Foundation of China (grant No. 82070684), the Guangdong Natural Science Fund for Outstanding Youth Scholars (grant No. 2020B1515020005), and the National Key Clinical Discipline.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-657/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-657/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-657/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-657/coif). LL reports that the study was supported by the National Natural Science Foundation of China (grant No. 82070684), and the Guangdong Natural Science Fund for Outstanding Youth Scholars (grant No. 2020B1515020005). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained from the Ethical Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (No. 2021ZSLYEC-325). The requirement for informed consent was waived for the retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Bozzetti FSCRINIO Working Group. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer 2009;17:279-84. [Crossref] [PubMed]
- Guner A, Cho M, Kim YM, et al. Prognostic Value of Postoperative Neutrophil and Albumin: Reassessment One Month After Gastric Cancer Surgery. Front Oncol 2021;11:633924. [Crossref] [PubMed]
- Lee JH, Park B, Joo J, et al. Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer 2018;21:913-24. [Crossref] [PubMed]
- Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901-14. [Crossref] [PubMed]
- Zhong S, Yan X, Wu Y, et al. Body mass index and mortality in prostate cancer patients: a dose-response meta-analysis. Prostate Cancer Prostatic Dis 2016;19:122-31. [Crossref] [PubMed]
- Kim LH, Doan P, He Y, et al. A Systematic Review and Meta-Analysis of the Significance of Body Mass Index on Kidney Cancer Outcomes. J Urol 2021;205:346-55. [Crossref] [PubMed]
- Komura N, Mabuchi S, Shimura K, et al. Significance of Pretreatment C-Reactive Protein, Albumin, and C-Reactive Protein to Albumin Ratio in Predicting Poor Prognosis in Epithelial Ovarian Cancer Patients. Nutr Cancer 2021;73:1357-64. [Crossref] [PubMed]
- Imamura T, Okamura Y, Sugiura T, et al. Clinical Significance of Preoperative Albumin-Bilirubin Grade in Pancreatic Cancer. Ann Surg Oncol 2021;28:6223-35. [Crossref] [PubMed]
- Miura K, Hamanaka K, Koizumi T, et al. Clinical significance of preoperative serum albumin level for prognosis in surgically resected patients with non-small cell lung cancer: Comparative study of normal lung, emphysema, and pulmonary fibrosis. Lung Cancer 2017;111:88-95. [Crossref] [PubMed]
- Guo X, Shao J, Zhai B, et al. Relationship and prognostic significance between preoperative serum albumin to globulin ratio and CT features of non-small cell lung cancer. Eur J Radiol 2020;128:109039. [Crossref] [PubMed]
- Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. [Crossref] [PubMed]
- Miyamoto R, Inagawa S, Sano N, et al. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol 2018;44:607-12. [Crossref] [PubMed]
- Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [Crossref] [PubMed]
- Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013;13:158. [Crossref] [PubMed]
- Kusunoki K, Toiyama Y, Okugawa Y, et al. Advanced Lung Cancer Inflammation Index Predicts Outcomes of Patients With Colorectal Cancer After Surgical Resection. Dis Colon Rectum 2020;63:1242-50. [Crossref] [PubMed]
- Topkan E, Ozdemir Y, Kucuk A, et al. Low Advanced Lung Cancer Inflammation Index Predicts Poor Prognosis in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Definitive Concurrent Chemoradiotherapy. J Oncol 2020;2020:3127275. [Crossref] [PubMed]
- Park YH, Yi HG, Lee MH, et al. Prognostic Value of the Pretreatment Advanced Lung Cancer Inflammation Index (ALI) in Diffuse Large B Cell Lymphoma Patients Treated with R-CHOP Chemotherapy. Acta Haematol 2017;137:76-85. [Crossref] [PubMed]
- Park SH, Lee S, Song JH, et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur J Surg Oncol 2020;46:620-5. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. 8th ed. New York: Springer, 2017.
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer 2019;19:672. [Crossref] [PubMed]
- Lu J, Xu BB, Xue Z, et al. Perioperative CRP: A novel inflammation-based classification in gastric cancer for recurrence and chemotherapy benefit. Cancer Med 2021;10:34-44. [Crossref] [PubMed]
- Taniai T, Haruki K, Furukawa K, et al. The novel index using preoperative C-reactive protein and neutrophil-to-lymphocyte ratio predicts poor prognosis in patients with pancreatic cancer. Int J Clin Oncol 2021;26:1922-8. [Crossref] [PubMed]
- Silva TH, Schilithz AOC, Peres WAF, et al. Neutrophil-lymphocyte ratio and nutritional status are clinically useful in predicting prognosis in colorectal cancer patients. Nutr Cancer 2020;72:1345-54. [Crossref] [PubMed]
- Ruan DY, Chen YX, Wei XL, et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol Rep (Oxf) 2021;9:560-70. [Crossref] [PubMed]
- Shigeto K, Kawaguchi T, Koya S, et al. Profiles Combining Muscle Atrophy and Neutrophil-to-Lymphocyte Ratio Are Associated with Prognosis of Patients with Stage IV Gastric Cancer. Nutrients 2020;12:1884. [Crossref] [PubMed]
- Parisi A, Cortellini A, Roberto M, et al. Weight loss and body mass index in advanced gastric cancer patients treated with second-line ramucirumab: a real-life multicentre study. J Cancer Res Clin Oncol 2019;145:2365-73. [Crossref] [PubMed]
- Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011;377:557-67. [Crossref] [PubMed]
- Egenvall M, Mörner M, Martling A, et al. Prediction of outcome after curative surgery for colorectal cancer: preoperative haemoglobin, C-reactive protein and albumin. Colorectal Dis 2018;20:26-34. [Crossref] [PubMed]
- Yin C, Toiyama Y, Okugawa Y, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin Nutr 2021;40:1130-6. [Crossref] [PubMed]
- Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer 2019;19:241. [Crossref] [PubMed]
- Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019;8:886-94. [Crossref] [PubMed]
- Wang F, Meng W, Wang B, et al. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196-202. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


