
The risk factors of biliary fistula after radical resection of perihilar cholangiocarcinoma in elderly patients and its influence on prognosis: a retrospective cohort study
Highlight box
Key findings
• The development of postoperative biliary fistula in elderly patients treated with radical resection of PHCC can seriously affect the prognosis of patients and reduce their survival and quality of life.
What is known and what is new?
• There are many independent risk factors for the development of biliary fistula after radical resection of PHCC. Numerous laboratory indicators are important in predicting the development of biliary fistula.
• The occurrence of biliary fistula after radical resection of PHCC significantly affects the prognosis of patients.
What are the implications, and what should change now?
• For elderly patients after radical resection of PHCC, the clinic should identify the relevant risk factors as early as possible and enact targeted measures to reduce the occurrence of postoperative biliary fistula, which will improve the prognosis of patients and prolong their survival.
Introduction
Cholangiocarcinoma (CCA) accounts for nearly 15% of all primary liver cancers worldwide and 2% of all cancer-related deaths (1). Perihilar cholangiocarcinoma (PHCC), which occurs between the level of the opening of the bile duct and the opening of the right and left hepatic duct secondary branches, is a common malignancy of the biliary system, accounting for 50–70% of all biliary tract tumors (1). According to the Bismuth-Corlette typing method (2), PHCC can be divided into the following types: type I, where the tumor is located at the bifurcation of the common hepatic duct and the left and right hepatic ducts are connected; type II, where the tumor is located at the confluence of the left and right hepatic ducts and the left and right hepatic ducts are not connected; type IIIa, where the tumor invades the right hepatic duct; type IIIb, where the tumor invades the left hepatic duct; and type IV, where both hepatic ducts are involved. PHCC is more likely to occur in middle-aged and elderly people aged 50 to 70 years old, and the physical condition of elderly patients is generally poorer based on the combination of some underlying diseases, and the postoperative complications of biliary fistula are more serious to them. The early symptoms of PHCC are non-specific, such as poor appetite, decreased appetite, aversion to greasy food, indigestion, and epigastric distention (3). Owing to its high malignancy, early lymph node metastasis, caudate lobe invasion, and nerve infiltration, and its special location (adjacent to the hepatic vessels and bile ducts), PHCC is difficult to treat and has a low cure rate is low (only about 30%) (4). With the improvement of China’s economic, development, and medical levels, the average life expectancy in the country has greatly increased. The proportion of the elderly population is gradually increasing but the trend of elderly patients with PHCC is also on the rise. Due to the influence of degenerative changes in physiological function and lowered immunity, the treatment of PHCC in elderly patients is more difficult, involving more postoperative complications and a shorter survival period.
Currently, complete surgical resection is the only treatment that is recognized to achieve a radical cure and maintain long-term survival (5). Since PHCC occurs in the core part of the biliary system and tends to extensively invade the surrounding blood vessels, nerve tissues, and major liver parenchyma, the surgical procedure is complex and technically difficult, and thus, open resection is mainly performed. Minimally invasive resection can be achieved only in limited cases (6). Local simple PHCC is only applicable to a small number of Bismuth-Corlette type I, and the vast majority of patients require radical surgery including liver resection. Radical surgery for PHCC consists of the following components: resection of the extrahepatic bile duct (including the right and left hepatic ducts at the confluence, the extrahepatic bile duct at the superior margin of the pancreas, the gallbladder, and the cystic duct), the segment of the liver lobe involved in the tumor, regional lymph node dissection (groups 8a, 8b, 9, 12, and 13a), the resection and reconstruction of the invasive hepatic artery and portal vein, as well as bile intestinal anastomosis (7,8).
Radical surgical liver resection is extensive, risky, and has a high incidence of postoperative complications, mainly including biliary fistula, liver failure, pleural effusion, abdominal infection, and adhesive bowel obstruction (9). Among these, as radical surgery involves a biliary incision, suturing, anastomosis, and drainage, poor biliary healing, and bile leakage may occur if there is a mistake or due to other patient-specific factors, which eventually leads to postoperative biliary fistula. According to the International Study Group for Liver Surgery (ISGLS) (10), postoperative biliary fistulas are graded as grade A fistulas, which are mild and hardly affect the prognosis of patients, while patients with grade B and C fistulas have a longer course and significantly impact the prognosis of patients. In recent years, although the medical level and surgical operation level have improved, the incidence of postoperative biliary fistula after radical surgery for PHCC is still as high as 31.5–38.9%, which is much higher than that of hepatectomy alone (4.8–14.0%) (11-14). Postoperative biliary fistula can have a serious impact on the quality of life (QOL) as well as the prognostic outcomes of patients, resulting in a considerable economic, physical, and mental health burden on patients and their families (15). Similarly, a previous study (16) pointed out that postoperative biliary fistula can also affect the long-term survival of patients. However, there are few reports on the risk factors affecting the development of postoperative biliary fistula and the impact of biliary fistula on the prognosis of patients. Therefore, there is a pressing need to further clarify the independent risk factors for the development of biliary fistula after radical surgery for PHCC and the impact of postoperative biliary fistula on the prognosis of elderly patients. Also, targeted preventive measures according to the stratification of relevant risk factors at an early stage are needed to provide a basis for preventing the occurrence of postoperative biliary fistula and improving the prognosis of elderly patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-34/rc).
Methods
Research participants
A total of 211 elderly patients who underwent radical surgery for PHCC at the First Affiliated Hospital of Nanjing Medical University from April 2016 to April 2021 were included in this study.
The inclusion criteria were as follows: (I) pathological diagnosis of PHCC; (II) patients aged ≥65 years, both sexes; (III) undergoing radical surgery including partial hepatectomy, extrahepatic bile duct resection, regional lymph node dissection, and Roux-en-Y biliary intestinal anastomosis; (IV) awake patients who were able to cooperate and complete the questionnaire; (V) complete clinicopathological data.
The exclusion criteria were as follows: (I) pathological diagnosis is not PHCC; (II) patients aged <65 years; (III) not having undergone radical surgery; (IV) suffering from mental illness or unable to communicate normally in language; (V) missing clinicopathological data (Figure 1).
According to whether the patients developed a biliary fistula and the grading of biliary fistula after surgery, we defined patients with grade A biliary fistula and no biliary fistula as the non-biliary fistula group, while grade B and C biliary fistulas were defined as the grade B and C biliary fistula groups, respectively. Finally, 211 elderly patients treated with radical surgery for PHCC were included in this study, according to the inclusion and exclusion criteria.
The incidence of biliary fistula was an important outcome indicator in this study. According to the review of previous studies (11-14), the incidence of biliary fistula in patients with PHCC is about 30%. The general rule of logistic regression requires a ratio of item number to sample size of 1:5–1:10 and our research factors are about 11. Therefore, the sample size of the study population planned for this study was 250 cases. There were 39 cases of actual attrition and missing part of cases, and 211 cases were finally counted.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. LC2021165). Informed consent was obtained from all patients.
General information questionnaire
The general information questionnaire included demographic data [e.g., gender, age, body mass index (BMI)] and clinical data [e.g., presence of hypertension and diabetes mellitus, Bismuth-Corlette staging, preoperative combined cholangitis or not, intraoperative hemorrhage or not, whether the surgery time was >6 h, the number of biliary anastomoses, whether the American Society of Anesthesiologists (ASA) score was ≥3, whether the serum albumin was <34 g/L, whether the specimen cut edge was R0, the extent of liver resection, preoperative biliary drainage or not, and whether the bile duct diameter was >1.5 cm].
Prognostic Score (PS)
The Eastern Cooperative Oncology Group (ECOG) PS is a common index for clinical evaluation of the daily activity level of cancer patients, which can predict the patients’ ability to tolerate treatment and reflect the quality of survival of cancer patients. The specific criteria are as follows: 0 point: normal mobility, no difference compared to the mobility before the disease; 1 point: free to walk and engage in light physical activities, including general household or office work but cannot engage in heavy physical activities; 2 points: free to walk and take care of themselves, but have lost the ability to work, and can get up and move around at least half of the day; 3 points: only partially able to take care of themselves, and more than half of the day is spent in bed or a wheelchair; 4 points: bedridden, unable to take care of themselves; 5 points: death.
Postoperative follow-up was conducted for 1 year by telephone, e-mail, and outpatient review. The last follow-up visit occurred in April 2022.
Karnofsky performance score (KPS)
The KPS is based on the patients’ condition, ability to perform normal activities, and degree of self-care. Higher scores indicate a better health status and ability to tolerate the side effects of treatment. The scores are as follows: 100: normal with no signs and symptoms; 90: able to perform normal activities with minor signs and symptoms; 80: barely able to perform normal activities with some signs and symptoms; 70: able to take care of himself/herself but cannot maintain normal life and work; 60: able to take care of himself/herself mostly but needs occasional help; 50: often needs care; 40: unable to take care of himself/herself and needs special care and assistance; 30: seriously unable to take care of himself/herself; 20: seriously ill, requiring hospitalization and active supportive treatment; 10: critically ill, near death; 0: death.
Postoperative follow-up was conducted for 1 year by telephone, e-mail, and outpatient review. The last follow-up visit occurred in April 2022.
QOL score
The QOL score is commonly used for oncology patients; it is a comprehensive assessment based on a total of 12 aspects, including appetite, sleep, spirit, fatigue, pain, family understanding and cooperation, colleagues’ understanding and cooperation, own knowledge of cancer, attitude toward treatment, daily life, side effects of treatment, and facial expression. It is calculated as a total score of 60, with a score ≤20 being very poor, 21–30 being poor, 31–40 being fair, 41–50 being good, and 51–60 being excellent.
Postoperative follow-up was conducted for 1 year by telephone, e-mail, and outpatient review. The last follow-up visit occurred in April 2022.
Kaplan-Meier survival curve
Postoperative follow-up was conducted for 1 year by telephone, e-mail, and outpatient review, which included a routine physical examination and tumor marker test. The patients were instructed to visit the hospital at any time if there was any discomfort. The follow-up ended with the patients’ death, and the follow-up deadline was April 2022.
Statistical analysis
The results of each scale were entered into the computer for score conversion, and statistical analysis was performed using SPSS 26 (IBM SPSS, USA). Measured data were expressed as the mean and standard deviation and count data were expressed as the frequency and percentage. Statistical analysis between the groups was performed using t-test, analysis of variance (ANOVA), and chi-square test, and factors influencing the occurrence of postoperative biliary fistula were analyzed by multiple logistic regression. Survival curves were plotted using the Kaplan-Meier method using Log-rank (Mantel-Cox) test. A two-sided P<0.05 was considered statistically significant.
Results
Baseline data
The baseline characteristics of the patients are shown in Table 1. A total of 211 elderly patients who underwent radical surgery for hilar CCA were included in this study. In the non-biliary fistula group, there were 110 (82.1%) patients aged <75 years and 24 (17.9%) patients aged ≥75 years, including 80 (59.7%) male patients and 54 (40.3%) female patients. In the biliary fistula group (including grade B and C biliary fistulas), there were 61 (79.2%) patients <75 years old and 16 (20.8%) patients ≥75 years old, including 50 (64.9%) male patients and 27 (35.1%) female patients. In the non-biliary fistula group, 37 (27.6%) patients had diabetes and 39 (29.1%) patients had hypertension. In the biliary fistula group, 39 (50.6%) patients had diabetes and 38 (49.4%) patients did not have diabetes; 20 (26.0%) patients had hypertension and 57 (74.0%) patients did not have hypertension. According to Bismuth-Corlette typing, in the non-biliary fistula group, 53 (39.6%) patients were type I, 31 (23.1%) patients were type II, 24 (17.9%) patients were type IIIa, 15 (11.2%) patients were type IIIb, and 11 (8.2%) patients were type IV. Meanwhile, in the biliary fistula group, 30 (39.0%) patients were type I, 13 (16.9%) patients were type II, 15 (19.5%) patients were type IIIa, 12 (15.6%) patients were type IIIb, and seven (9.0%) patients were type IV. As for the extent of liver resection, in the non-biliary fistula group, 22 (16.4%) patients had a small area of liver resected, 65 (48.5%) patients had a triple area resected, and 47 (35.1%) patients had an extended hemi-hepatectomy; in the biliary fistula group, 18 (23.4%) patients had a small area of liver resected, 33 (42.9%) patients had a triple area resected, and 26 (33.7%) patients had an extended hemi-hepatectomy. Moreover, in the non-biliary fistula group, 20 (14.9%) patients had preoperative biliary ductitis, 57 (42.5%) patients had preoperative biliary drainage, 24 (17.9%) patients had intraoperative major bleeding, and 29 (21.6%) patients had ≥2 biliary anastomoses; while in the biliary fistula group, 29 (37.7%) patients had preoperative biliary ductitis, 50 (64.9%) patients had preoperative biliary drainage, 23 (29.9%) patients had intraoperative major bleeding, and 40 (51.9%) patients had biliary anastomoses ≥2.
Table 1
| Item | Biliary fistulas group, N (%) | No biliary fistula group, N (%) | χ2 | P |
|---|---|---|---|---|
| Gender | 0.566 | 0.452 | ||
| Male | 50 (64.9) | 80 (59.7) | ||
| Female | 27 (35.1) | 54 (40.3) | ||
| Age (years) | 0.262 | 0.609 | ||
| <75 | 61 (79.2) | 110 (82.1) | ||
| ≥75 | 16 (20.8) | 24 (17.9) | ||
| BMI, kg/m2 | 23.894 | 0.000 | ||
| <18 | 3 (3.9) | 12 (9.0) | ||
| 18–24 | 32 (41.6) | 93 (69.4) | ||
| >24 | 42 (54.5) | 29 (21.6) | ||
| Diabetes | 11.262 | 0.001 | ||
| Yes | 39 (50.6) | 37 (27.6) | ||
| No | 38 (49.4) | 97 (72.4) | ||
| Hypertension | 0.238 | 0.626 | ||
| Yes | 20 (26.0) | 39 (29.1) | ||
| No | 57 (74.0) | 95 (70.9) | ||
| Type of Bismuth-Corlette | 1.767 | 0.778 | ||
| I | 30 (39.0) | 53 (39.6) | ||
| II | 13 (16.9) | 31 (23.1) | ||
| IIIa | 15 (19.5) | 24 (17.9) | ||
| IIIb | 12 (15.6) | 15 (11.2) | ||
| IV | 7 (9.0) | 11 (8.2) | ||
| Preoperative cholangitis | 14.179 | 0.000 | ||
| Yes | 29 (37.7) | 20 (14.9) | ||
| No | 48 (62.3) | 114 (85.1) | ||
| Major bleeding during the operation | 4.040 | 0.044 | ||
| Yes | 23 (29.9) | 24 (17.9) | ||
| No | 54 (70.1) | 110 (82.1) | ||
| Operation time >6 h | 4.891 | 0.027 | ||
| Yes | 24 (31.2) | 24 (17.9) | ||
| No | 53 (68.8) | 110 (82.1) | ||
| Number of biliary anastomoses | 20.408 | 0.000 | ||
| ≤1 | 37 (48.1) | 105 (78.4) | ||
| ≥2 | 40 (51.9) | 29 (21.6) | ||
| ASA score ≥3 | 0.759 | 0.384 | ||
| Yes | 45 (58.4) | 70 (52.2) | ||
| No | 32 (41.6) | 64 (47.8) | ||
| Alb | 4.684 | 0.030 | ||
| <34 g/L | 35 (45.5) | 41 (30.6) | ||
| ≥34 g/L | 42 (54.5) | 93 (69.4) | ||
| Cut edge of specimen | 0.003 | 0.958 | ||
| R0 | 56 (72.7) | 97 (72.4) | ||
| R1 | 21 (27.3) | 37 (27.6) | ||
| Scope of liver resection | 1.609 | 0.447 | ||
| ≤three hepatic segments | 18 (23.4) | 22 (16.4) | ||
| Fall in between three hepatic segments and hemihepatic | 33 (42.9) | 65 (48.5) | ||
| Hemihepatic or enlarged hemihepatic | 26 (33.7) | 47 (35.1) | ||
| Preoperative biliary drainage | 9.815 | 0.002 | ||
| Yes | 50 (64.9) | 57 (42.5) | ||
| No | 27 (35.1) | 77 (57.5) | ||
| Bile duct diameter >1.5 cm | 4.798 | 0.028 | ||
| Yes | 30 (39.0) | 33 (24.6) | ||
| No | 47 (61.0) | 101 (75.4) |
PHCC, perihilar cholangiocarcinoma; BMI, body mass index; ASA, American Society of Anesthesiologists; Alb, albumin.
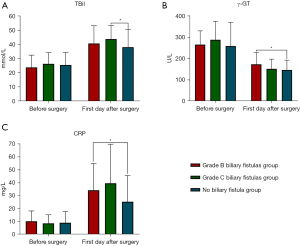
Comparison of the laboratory indexes before and on the first day after radical surgery for PHCC among the three groups
According to ANOVA, there were no differences in the preoperative laboratory indexes among the non-biliary fistula group, the grade B biliary fistula group, and the grade C biliary fistula group, while postoperative the laboratory indexes of γ-glutamyl transpeptidase (γ-GT), TBil (total bilirubin), and C-reactive protein (CRP) were statistically significant among the three groups (P<0.05). Further two-by-two comparison revealed that CRP indexes were significantly different between the non-biliary fistula group and the grade B or C biliary fistula groups (P<0.05), and CRP indexes were significantly lower in the non-biliary fistula group than in the grade B or C biliary fistula groups on the first day after surgery. Meanwhile, γ-GT were significantly lower in the non-biliary fistula group than in the grade B biliary fistula group and there was a significant difference between them (P<0.05); TBil was also the lowest in the non-biliary fistula group, and there was a significant difference between the non-biliary fistula group and the grade C biliary fistula group (P<0.05) (Tables 2,3, Figure 2).
Table 2
| Item | Before surgery, () | First day after surgery, () | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade B biliary fistulas group | Grade C biliary fistulas group | Non-biliary fistula group | F | P | Grade B biliary fistulas group | Grade C biliary fistulas group | Non-biliary fistula group | F | P | ||
| PLT (×109 U/L) | 221.82±71.549 | 214.44±82.354 | 210.02±73.287 | 0.429 | 0.651 | 193.07±157.972 | 174.22±51.114 | 168.10±58.528 | 1.336 | 0.265 | |
| APTT (s) | 30.00±5.808 | 29.84±5.908 | 29.63±5.374 | 0.079 | 0.924 | 28.69±6.175 | 27.69±4.439 | 27.72±4.728 | 0.670 | 0.513 | |
| ALT (U/L) | 47.02±21.856 | 52.13±25.108 | 46.66±23.742 | 0.709 | 0.493 | 613.80±135.145 | 614.50±161.945 | 644.83±141.778 | 0.799 | 0.451 | |
| AST (U/L) | 49.38±23.856 | 45.59±17.318 | 46.22±23.736 | 0.371 | 0.690 | 639.11±141.666 | 606.31±179.697 | 598.88±184.739 | 0.886 | 0.414 | |
| γ-GT (U/L) | 265.76±64.099 | 288.38±86.252 | 259.22±110.422 | 1.124 | 0.327 | 173.33±55.469 | 152.13±45.429 | 146.45±46.399 | 5.221 | 0.006 | |
| ALP (U/L) | 192.60±58.247 | 214.59±58.620 | 205.63±65.451 | 1.233 | 0.294 | 101.87±28.343 | 101.41±32.784 | 100.35±30.279 | 0.049 | 0.952 | |
| TBil (mmol/L) | 23.84±8.623 | 26.28±7.956 | 25.49±8.942 | 0.857 | 0.426 | 40.64±12.419 | 43.75±9.544 | 38.02±12.472 | 3.189 | 0.043 | |
| ChE (kU/L) | 5.431±0.5846 | 5.513±0.6695 | 5.465±0.7204 | 0.132 | 0.877 | 4.044±0.3787 | 4.044±0.4173 | 4.069±0.3772 | 0.106 | 0.900 | |
| CRP (mg/L) | 10.324±7.8809 | 8.581±6.6950 | 9.129±8.6640 | 0.499 | 0.608 | 34.187±20.6262 | 39.566±30.2534 | 25.310±20.2025 | 6.750 | 0.001 | |
PHCC, perihilar cholangiocarcinoma; PLT, platelet; APTT, activated partial thromboplastin time; ALT, alanine transaminase; AST, aspartate transaminase; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; TBil, total bilirubin; ChE, Cholinesterase; CRP, C-reactive protein.
Table 3
| Item | γ-GT (U/L) | TBil (mmol/L) | CRP (mg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | Mean range | 95% CI | P | Mean range | 95% CI | P | Mean range | 95% CI | ||||||
| Upper | Lower | Upper | Lower | Upper | Lower | |||||||||
| Grade B and C biliary fistulas group | 0.195 | 21.208 | 49.37 | −6.96 | 0.523 | −3.106 | 3.01 | −9.22 | 0.770 | −5.3790 | 9.851 | −20.609 | ||
| Non-biliary fistula group and grade B biliary fistulas group | 0.014 | −26.886 | −4.37 | −49.40 | 0.534 | −2.622 | 2.61 | −7.85 | 0.042 | −8.8770 | −0.241 | −17.513 | ||
| Non-biliary fistula group and grade C biliary fistulas group | 0.896 | −5.677 | 16.53 | −27.89 | 0.017 | −5.728 | −0.81 | −10.65 | 0.046 | −14.2559 | −0.203 | −28.309 | ||
PHCC, perihilar cholangiocarcinoma; γ-GT, γ-glutamyl transpeptidase; TBil, total bilirubin; CRP, C-reactive protein; CI, confidence interval.
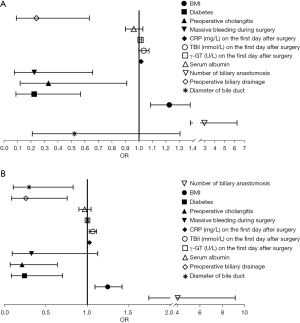
Multiple logistics regression analysis between the non-biliary fistula group and grade B and C biliary fistula group
Multiple logistic regression analysis showed that BMI, diabetes, preoperative cholangitis, number of biliary anastomosis and preoperative biliary drainage were common independent risk factors for both grade B and C biliary fistulas; while intraoperative major bleeding and γ-GT on the first postoperative day were independent risk factors for the grade B biliary fistulas. Also, bile duct diameter, CRP, and TBil on the first postoperative day were independent risk factors for grade C biliary fistulas (Table 4, Figure 3).
Table 4
| Item | Related factor | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Upper | Lower | |||||||
| Non-biliary fistula group and grade B biliary fistulas group | BMI | 0.202 | 0.061 | 10.985 | 0.001 | 1.224 | 1.379 | 1.086 |
| Diabetes or not | −1.507 | 0.479 | 9.901 | 0.002 | 0.222 | 0.566 | 0.087 | |
| Preoperative cholangitis or not | −1.116 | 0.521 | 4.591 | 0.032 | 0.328 | 0.909 | 0.118 | |
| Major intraoperative bleeding or not | −1.500 | 0.550 | 7.424 | 0.006 | 0.223 | 0.656 | 0.076 | |
| CRP on the first day after surgery | 0.015 | 0.009 | 2.794 | 0.095 | 1.015 | 1.033 | 0.997 | |
| TBil on the first day after surgery | 0.035 | 0.019 | 3.484 | 0.062 | 1.035 | 1.074 | 0.998 | |
| γ-GT on the first day after surgery | 0.11 | 0.004 | 6.214 | 0.013 | 1.011 | 1.020 | 1.002 | |
| Alb | −0.039 | 0.034 | 1.320 | 0.251 | 0.961 | 1.028 | 0.899 | |
| Number of biliary enterostomy | 1.077 | 0.385 | 7.817 | 0.005 | 2.935 | 6.242 | 1.380 | |
| Preoperative biliary drainage or not | −1.433 | 0.497 | 8.305 | 0.004 | 0.239 | 0.632 | 0.090 | |
| Bile duct diameter >1.5 cm or not | −0.652 | 0.468 | 1.941 | 0.164 | 0.521 | 1.304 | 0.208 | |
| No biliary fistula group and grade C biliary fistulas group | BMI | 0.219 | 0.067 | 10.697 | 0.001 | 1.245 | 1.419 | 1.092 |
| Diabetes or not | −1.410 | 0.539 | 6.852 | 0.009 | 0.244 | 0.702 | 0.085 | |
| Preoperative cholangitis or not | −1.544 | 0.559 | 7.625 | 0.006 | 0.214 | 0.639 | 0.071 | |
| Major intraoperative bleeding or not | −1.116 | 0.629 | 3.145 | 0.076 | 0.328 | 1.125 | 0.095 | |
| CRP on the first day after surgery | 0.025 | 0.010 | 6.424 | 0.011 | 1.026 | 1.046 | 1.006 | |
| TBil on the first day after surgery | 0.064 | 0.021 | 9.002 | 0.003 | 1.066 | 1.112 | 1.022 | |
| γ-GT on the first day after surgery | 0.002 | 0.005 | 0.129 | 0.720 | 1.002 | 1.012 | 0.992 | |
| Alb | −0.032 | 0.039 | 0.694 | 0.405 | 0.968 | 1.045 | 0.898 | |
| Number of biliary enterostomy | 1.385 | 0.424 | 10.676 | 0.001 | 3.995 | 9.170 | 1.741 | |
| Preoperative biliary drainage or not | −1.348 | 0.547 | 6.080 | 0.014 | 0.260 | 0.758 | 0.089 | |
| Bile duct diameter >1.5 cm or not | −1.207 | 0.520 | 5.387 | 0.020 | 0.299 | 0.829 | 0.108 | |
PHCC, perihilar cholangiocarcinoma; BMI, body mass index; CRP, C-reactive protein; TBil, total bilirubin; γ-GT, γ-glutamyl transpeptidase; Alb, albumin; SE, standard error; OR, odds ratio; CI, confidence interval.
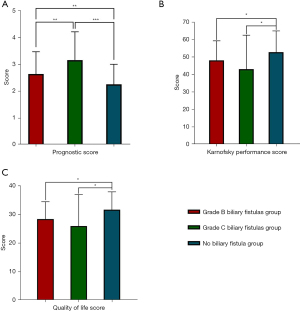
Comparison of the PS, KPS, and QOL Scores among the three groups after radical resection of PHCC
According to ANOVA, there were significant differences in the PS, KPS, and QOL scores among the three groups after radical surgery for PHCC (P<0.05). Further two-by-two comparison revealed significant differences between the two groups in terms of the PS scores, and the non-biliary fistula group had lower scores than the grade B or C biliary fistula group. As for the KPS and QOL scores, the non-biliary fistula group was significantly higher than the grade B or C biliary fistula group, and the differences were statistically significant (P<0.05) (Tables 5,6, Figure 4).
Table 5
| Item | PS | KPS | QOL score |
|---|---|---|---|
| Grade B biliary fistulas group, () | 2.64±0.830 | 48.22±11.137 | 28.44±6.055 |
| Grade C biliary fistulas group, () | 3.16±1.051 | 43.13±19.417 | 25.97±11.020 |
| Non-biliary fistula group, () | 2.25±0.753 | 52.84±12.177 | 31.69±6.289 |
| F | 16.877 | 7.622 | 9.840 |
| P | 0.000 | 0.001 | 0.000 |
PS, Prognostic Score; KPS, Karnofsky performance score; QOL, quality of life; PHCC, perihilar cholangiocarcinoma.
Table 6
| Item | PS | KPS | QOL score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | Mean range | 95% CI | P | Mean range | 95% CI | P | Mean range | 95% CI | ||||||
| Upper | Lower | Upper | Lower | Upper | Lower | |||||||||
| Grade C and B biliary fistulas group | 0.008 | 0.512 | 0.89 | 0.14 | 0.503 | −4.875 | 4.59 | −14.34 | 0.587 | −2.476 | 2.85 | −7.80 | ||
| Grade B biliary fistulas group and non-biliary fistula group | 0.006 | 0.391 | 0.67 | 0.11 | 0.048 | −4.836 | −0.03 | −9.65 | 0.009 | −3.242 | −0.67 | −5.81 | ||
| Grade C biliary fistulas group and non-biliary fistula group | 0.000 | 0.903 | 1.22 | 0.58 | 0.030 | −9.711 | −0.73 | −18.69 | 0.023 | −5.718 | −0.65 | −10.78 | ||
PS, Prognostic Score; KPS, Karnofsky performance score; QOL, quality of life; PHCC, perihilar cholangiocarcinoma.
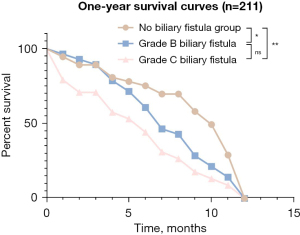
Comparison of the Kaplan-Meier survival curves between the Grade B, C biliary fistula group and non-biliary fistula group
The follow-up lasted for 1 year, and the difference in cumulative survival function between the non-biliary fistula group and the grade B or C biliary fistula group was statistically significant (P<0.05). However, there was no significant difference in the survival function between the grade B biliary fistula group and the grade C biliary fistula group (Figure 5).
Discussion
PHCC is a biliary tumor that invades the proximal extrahepatic bile duct, accounting for 50–70% of all biliary tract tumors (1). The treatment and management of PHCC remain challenging, especially considering its aggressive nature and complex anatomical relationships (17). The current treatment of patients with PHCC is mainly based on hepatic resection and extrahepatic biliary resection, and despite the increasing technological advances in medical treatment and experience in hepatic resection, the postoperative complication rates (4.09–47.7%) and mortality rates (0.24–9.7%) remain high (18). Common postoperative complications include fever, bleeding, biliary fistula, liver failure, pleural effusion, and subdiaphragmatic infection, with biliary fistula exhibiting a high incidence (19-22). The common causes of postoperative biliary fistula are as follows: (I) truncation of the distal duct of the residual liver, which is the most common cause; (II) leakage from the bile duct-intestinal anastomosis, or incomplete suturing around the t-tube; (III) bile duct injury due to improper surgical technique. In addition, biliary fistula has adverse effects on both the near and long-term prognosis of patients (12,13,23).
In this study, the incidence of biliary fistula complicating radical surgery for PHCC was 36.49%. There were significant differences (P<0.05) between the biliary fistula group and the non-biliary fistula group in terms of BMI and whether they had diabetes or preoperative cholangitis. Among these, diabetes, as a common underlying disease in elderly patients, leads to suboptimal tolerance of the patient’s body, as diabetes and PHCC are both metabolic diseases. High blood sugar, as a source of energy, leads to the continuous growth and reproduction of cancer cells, which aggravates the degree of impaired liver function; the continued impairment of liver function in turn aggravates diabetes, resulting in glucose metabolism disorders and decreased glucose tolerance. This leads to further aggravation of diabetes, thus forming a vicious circle and seriously damaging the health of patients (24). Therefore, early identification, diagnosis, and intervention for high-risk patients in the clinical treatment process will provide positive clinical outcomes.
In this study, we retrospectively analyzed the independent risk factors for biliary fistula in 211 elderly patients after radical surgery for PHCC and showed that BMI, diabetes, the number of biliary anastomoses, whether the patient had preoperative cholangitis, and whether preoperative biliary drainage was present were common independent risk factors for grade B and C biliary fistulas, according to the multiple logistic regression analysis. Preoperative cholangitis can contribute to the formation of small abscesses and edema in the bile duct wall, which, together with local trauma and irritation caused by the surgical procedure, makes postoperative stump healing more difficult, thus increasing the risk of infection and making patients more susceptible to postoperative biliary fistula (25). Cassani et al. (26) pointed out that biliary drainage could effectively reduce the incidence of cholangitis and improve the survival of PHCC patients. Also, preoperative biliary drainage is an irreplaceable tool for the treatment of acute cholangitis, as it can both monitor the pathogenesis and guide the selection of antibiotics.
In addition, the number of biliary anastomoses correlates with the risk of postoperative biliary fistula via biliary anastomosis. Hemihepatic and triple resection are the most commonly used surgical approaches for hilar CCA, with the advantages of easy access to R0 resection, absence of large hepatic branches in the liver section, a relatively small number of bile duct openings [1–2], and simpler biliary anastomosis (27). Several studies have confirmed the safety and effectiveness of extensive hepatic resection (28-30). Therefore, if the condition allows, choosing a wide range of hepatectomy can effectively reduce the number of biliary anastomoses and the difficulty of biliary anastomosis, thereby reducing the risk of biliary fistula, especially clinically relevant biliary fistula after surgery for PHCC. This study also found that intraoperative major bleeding and first-day postoperative γ-GT were independent risk factors for grade B biliary fistulas. In a retrospective analysis of 505 hepatectomy cases, Yoshioka et al. (31) found that intraoperative bleeding ≥775 mL was an independent risk factor for postoperative biliary fistula. Yamashita et al. (22) showed that in high-risk procedures, there may be many opportunities to disrupt the Glisson sheath around the hepatoportal, leading to intraoperative bleeding or large surgical wounds, and damage to the small bile ducts in the separated segment of the liver may lead to non-connection with the main bile duct, and thus, biliary leakage. Atahan et al. (32) showed that γ-GT plays an important role in predicting postoperative “occult biliary fistula” in patients with hepatic echinococcosis. However, the exact mechanism of the association between γ-GT and postoperative biliary fistula has not been reported in the literature and needs to be further investigated.
Finally, the present study also identified that CRP and TBil on the first postoperative day, as independent risk factors for grade C biliary fistula, are sensitive indicators for the diagnosis of early inflammatory infections. They are produced by the induction of inflammatory cytokines, mainly in hepatocytes. Under normal conditions, CRP is present in trace amounts in the serum of healthy individuals, which has the ability to activate the complement and promote the phagocytosis of granulocytes and macrophages. CRP levels increase significantly when there is acute inflammation, trauma, or infarction in the organism, and it will decrease rapidly after the clearance of inflammation and tissue cells (33). According to the statistical results of this study, the postoperative CRP levels in all groups were significantly higher than the preoperative levels, which could be attributable to the fact that patients underwent radical surgery for PHCC and had considerable liver trauma and bile duct injury, leading to the occurrence of local inflammatory reactions, thus increasing the risk of postoperative biliary fistula. The postoperative TBil levels were also significantly higher than the preoperative levels, which may be due to the bile duct injury that caused the patient's bile to leak into the abdominal cavity and the abdominal absorption into the blood, resulting in increased TBil in the blood after surgery.
According to the PS, KPS, and QOL scores, this study found that there was a significant difference between the grade B biliary fistula group, the grade C biliary fistula group, and the non-biliary fistula group (P<0.05). This indicates that the occurrence of biliary fistula after radical surgery for PHCC would have a significant impact on the prognosis of patients, especially in terms of their physical status, daily activities, and QOL. Therefore, we should focus on high-risk patients during clinical treatment and perform early and effective treatment measures based on the relevant risk factors to reduce the occurrence of biliary fistula, so as to improve the prognosis and postoperative survival quality of patients.
Studies (34,35) have reported that the 1-year survival rate after radical surgery for PHCC is around 50–60%; the results in the present study were higher than these previously reported findings, indicating that the survival rate of patients after radical surgery for PHCC is still low, and the occurrence of biliary fistula after surgery seriously affects the prognosis of patients.
Finally, the main drawback of this study was the small number of cases counted and the relatively short follow-up period due to time and manpower limitations. Therefore, it is recommended that longer follow-up periods should be applied in future studies.
Conclusions
The incidence of postoperative biliary fistula remains high in elderly patients treated with radical surgery for PHCC, seriously affecting the prognosis of patients and reducing their survival and QOL. Therefore, individualized preventive measures should be performed according to the clinical characteristics and risk factors of patients to hinder the possibility of postoperative biliary fistula, which will improve their prognosis and prolong their life expectancy.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 82273066).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-34/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-34/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. LC2021165). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88. [Crossref] [PubMed]
- Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31-8. [Crossref] [PubMed]
- Saxena A, Chua TC, Chu FC, et al. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg 2011;202:310-20. [Crossref] [PubMed]
- Hosokawa I, Shimizu H. ASO Author Reflections: Left Trisectionectomy for Bismuth-Corlette Type IV Perihilar Cholangiocarcinoma with Left-Sided Predominance. Ann Surg Oncol 2020;27:2387-8. [Crossref] [PubMed]
- Zhang M, Liu L, Zheng M, et al. Progress on surgical treatment of hilar cholangiocarcinoma. Journal of Hepatopancreatobiliary Surgery 2020;32:116-21.
- Ellis RJ, Soares KC, Jarnagin WR. Preoperative Management of Perihilar Cholangiocarcinoma. Cancers (Basel) 2022;14:2119. [Crossref] [PubMed]
- Mizuno T, Ebata T, Yokoyama Y, et al. Combined Vascular Resection for Locally Advanced Perihilar Cholangiocarcinoma. Ann Surg 2022;275:382-90. [Crossref] [PubMed]
- Nagino M, Nimura Y, Nishio H, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg 2010;252:115-23. [Crossref] [PubMed]
- Daradkeh S. A case series of hilar cholangiocarcinoma: A single surgeon experience over 20-years. Ann Med Surg (Lond) 2021;62:239-43. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Benson AB 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-91. [Crossref] [PubMed]
- Ito A, Ebata T, Yokoyama Y, et al. Ethanol ablation for refractory bile leakage after complex hepatectomy. Br J Surg 2018;105:1036-43. [Crossref] [PubMed]
- Braunwarth E, Primavesi F, Göbel G, et al. Is bile leakage after hepatic resection associated with impaired long-term survival? Eur J Surg Oncol 2019;45:1077-83. [Crossref] [PubMed]
- Spetzler VN, Schepers M, Pinnschmidt HO, et al. The incidence and severity of post-hepatectomy bile leaks is affected by surgical indications, preoperative chemotherapy, and surgical procedures. Hepatobiliary Surg Nutr 2019;8:101-10. [Crossref] [PubMed]
- Zhang ZG. Clinical Analysis of Bile Leakage after Biliary Surgery. Medical Information 2018;31:189-90.
- Tzedakis S, Sindayigaya R, Dhote A, et al. Perihilar cholangiocarcinoma: What the radiologist needs to know. Diagn Interv Imaging 2022;103:288-301. [Crossref] [PubMed]
- Soares KC, Jarnagin WR. The Landmark Series: Hilar Cholangiocarcinoma. Ann Surg Oncol 2021;28:4158-70. [Crossref] [PubMed]
- Jin S, Fu Q, Wuyun G, et al. Management of post-hepatectomy complications. World J Gastroenterol 2013;19:7983-91. [Crossref] [PubMed]
- Lee CC, Chau GY, Lui WY, et al. Risk factors associated with bile leakage after hepatic resection for hepatocellular carcinoma. Hepatogastroenterology 2005;52:1168-71.
- Sadamori H, Yagi T, Matsuda H, et al. Risk factors for major morbidity after hepatectomy for hepatocellular carcinoma in 293 recent cases. J Hepatobiliary Pancreat Sci 2010;17:709-18. [Crossref] [PubMed]
- Bhattacharjya S, Puleston J, Davidson BR, et al. Outcome of early endoscopic biliary drainage in the management of bile leaks after hepatic resection. Gastrointest Endosc 2003;57:526-30. [Crossref] [PubMed]
- Yamashita Y, Hamatsu T, Rikimaru T, et al. Bile leakage after hepatic resection. Ann Surg 2001;233:45-50. [Crossref] [PubMed]
- Martin AN, Narayanan S, Turrentine FE, et al. Clinical Factors and Postoperative Impact of Bile Leak After Liver Resection. J Gastrointest Surg 2018;22:661-7. [Crossref] [PubMed]
- Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology 2013;57:964-73. [Crossref] [PubMed]
- Wang Y, Fu W, Tang Z, et al. Effect of preoperative cholangitis on prognosis of patients with hilar cholangiocarcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e12025. [Crossref] [PubMed]
- Cassani LS, Chouhan J, Chan C, et al. Biliary Decompression in Perihilar Cholangiocarcinoma Improves Survival: A Single-Center Retrospective Analysis. Dig Dis Sci 2019;64:561-9. [Crossref] [PubMed]
- Uysal F, Obuz F, Uçar A, et al. Anatomic variations of the intrahepatic bile ducts: analysis of magnetic resonance cholangiopancreatography in 1011 consecutive patients. Digestion 2014;89:194-200. [Crossref] [PubMed]
- Di Martino M, Dorcaratto D, Primavesi F, et al. Liver resection in elderly patients with extensive CRLM: Are we offering an adequate treatment? A propensity score matched analysis. Eur J Surg Oncol 2022;48:1331-8. [Crossref] [PubMed]
- Ebata T, Mizuno T, Yokoyama Y, et al. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. Br J Surg 2018;105:829-38. [Crossref] [PubMed]
- Li C, Zhu A. Application of Image Fusion in Diagnosis and Treatment of Liver Cancer. Applied Sciences 2020; [Crossref]
- Yoshioka R, Saiura A, Koga R, et al. Predictive factors for bile leakage after hepatectomy: analysis of 505 consecutive patients. World J Surg 2011;35:1898-903. [Crossref] [PubMed]
- Atahan K, Küpeli H, Deniz M, et al. Can occult cystobiliary fistulas in hepatic hydatid disease be predicted before surgery? Int J Med Sci 2011;8:315-20. [Crossref] [PubMed]
- Nehring S M, Goyal A, Patel BC. C Reactive Protein[M]. StatPearls. Treasure Island (FL); StatPearls Publishing; July 18, 2022.
- Nakanishi Y, Hirano S, Okamura K, et al. Clinical and oncological benefits of left hepatectomy for Bismuth type I/II perihilar cholangiocarcinoma. Surg Today 2022;52:844-52. [Crossref] [PubMed]
- Zhang Y, Dou C, Wu W, et al. Total laparoscopic versus open radical resection for hilar cholangiocarcinoma. Surg Endosc 2020;34:4382-7. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



