
Therapeutic response analysis for patients with adenosquamous carcinoma of the gallbladder: data analysis based on the Surveillance, Epidemiology, and End Results (SEER) database
Highlight box
Key findings
• The therapeutic benefit of different treatment for patients with GBASC was assessed, and the optimal clinical measures for GBASC patients was determined.
What is known and what is new?
• GBASC is a rare subtype of gallbladder carcinoma, with different clinical characteristics from those of GBAC.
• A larger sample size of GBASC patient was analyzed, the therapeutic response and the prognostic factors of GBASC patients were determined.
What is the implication, and what should change now?
• Intraoperative lymph node dissection during surgery is preferred when treating patients with early-stage GBASC; surgical treatment (particularly radical surgery), lymph node dissections that are more comprehensive, radiotherapy, and chemotherapy are optimal treatments for advanced patients with GBASC. Surgery, lymph node dissection, radiation, chemotherapy, age, race, and AJCC stage can be used as prognostic factor for GBASC patients.
Introduction
Cancer of the gallbladder is the most prevalent type of malignancy affecting the biliary tract, with annual incidence ranging from 0.35/100,000 to 3.0/100,000 worldwide (1). More than 90–95% of cases of gallbladder cancer (GBC) are caused by the most common histologic subtype, adenocarcinoma (AC), which has been extensively researched regarding its clinicopathologic features and survival outcomes (2,3), while adenosquamous/squamous cells (2–10%) and undifferentiated cells (2–7%) constitute the remaining number of cases (4-7).
There is presently no standardized definition for adenosquamous carcinoma of the gallbladder (GBASC), and it is regularly recognized in the literature as having tumors with both glandular and squamous features. Several criteria were employed in various studies to determine the extent of squamous differentiation, including any squamous component (8), more than 10% squamous differentiation (9-11), more than 25% squamous differentiation (4), and more than 30% squamous differentiation (5,6). It is common for researchers to include squamous differentiation in the GBASC cohort since there are much fewer cases of GBASC than there are of mixed-difference tumors in the breasts and pancreas (12). For this study, we thus defined GBASC as including any component of squamous differentiation.
Due to the rarity of GBASC, only a few studies have explored the efficacy of different treatment modalities and the prognostic factors of patients with GBASC, and of those that were conducted, most were single-center case studies while a few were population studies (4-6,13-17). In the available research, GBASC was observed to be more prevalent in women (4,13) and was associated with greater tumor size (6,9,13,14,17-19), poor differentiation (13-15,17), shorter survival (13,14), an advanced stage at presentation (5,6,13), more aggressive invasiveness to adjacent viscera (6), and inconsistent findings with regard to other distant organs and lymph node metastasis (5,8,15-17). In terms of the therapy modality, surgical resection, especially radical surgery, was considered the effective treatment for patients with GBASC (5,6,8,13,17), while chemotherapy and radiotherapy could also improve the prognosis to some extent (13). Although some of these correlations are well-supported or consistent across the research, some issues related to GBASC still need to be addressed: owing to the rarity of GBASC, the research to evaluate the treatment response of patients with GBASC was dearth, and the most appropriate clinical measures for treating patients with GBASC in the different stages must be determined; moreover, the clinicopathological variables associated with prognosis of GBASC were still unclear.
Researchers searched the Surveillance, Epidemiology, and End Results (SEER) database for information on a large number of patients with GBASC, and conducted a retrospective study on the cohort of them to examine the curative benefits of the various treatment options for patients with GBASC at various stages and to investigate the risk factors affecting the prognosis of GBASC to assist physicians in determining clinical decisions about these patients’ treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1292/rc).
Methods
Data source
The SEER program of the National Cancer Institute (NCI) was constructed from 21 population-based cancer registries to provide information on roughly 34.6% of the US population. In this study, an independent cohort of GBASC patients was retrieved, and all the relevant demographic and clinical characteristics were reviewed and noted by trained investigators who were blind to the predictor variables of our research. The records of patient survival information were not determined by the subjective judgment of blinded investigators but depended on the death certificates. Data on patients with a primary cancer site inside the gallbladder were extracted using topographical codes from the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3: C23.9) using SEER Stat 8.4.0. Only patients diagnosed between the year 1975 and 2018 with the ICD-O-3 histology/behavior codes 8070/3, 8071/3, 8074/3, 8560/3, and 8570/3 were included in this study to concentrate on the GBASC. During the follow up, the cancer data collection was periodically performed by identifying patients in the medical institution, and cancer registries pulled cancer information from the medical records. All patients were followed up until they were dead or to the date of last follow-up in November 2020, any lost to follow-up case was exclude from the study. Stage I and stage II were considered early stages as per the American Joint Committee on Cancer (AJCC) staging concept of 2004, whereas stage III and stage IV were considered advanced stages. Only patients with a diagnosis confirmed by positive histology at pathological analysis or positive exfoliative cytology were included. Patients with any of the following conditions were excluded: (I) GBASC as secondary cancer; (II) patients without definitive information on pathological type, degree of differentiation, or metastasis; (III) incomplete patient follow-up information; and (IV) patients with autopsy confirmation. To increase the authority and generalizability of our study, the maximum quantity of patient information in the database that met the above-mentioned criteria was included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Demographic and clinical features including gender (male, female), age (65 years old), ethnicity (American Indian/Alaska Native, Asian or Pacific Islander, Black, White), family status (divorced, married, single), extent of operation (no surgery, radical surgery, nonradical surgery), lymph node dissection (removal of 1 to 3 regional lymph nodes, 4 or more regional lymph nodes, none), receipt of chemotherapy (yes, no), receipt of radiotherapy (yes, no), cancer grade (well differentiated; moderately differentiated; poorly differentiated; undifferentiated, anaplastic), pathological primary tumor T stage (T1–T2, T3–T4), M stage (M0, M1), and N stage (N0, N1) according to the AJCC Cancer Staging Manual, 6th edition, life expectancy and cause of death could be discerned from data in the SEER database. Cases with the above information missing were excluded from analysis. In this paper, “radical surgery” refers to the whole excision of the gallbladder with resection in continuity (partial or total removal) with adjacent organs, whereas “nonradical surgery” refers to the removal of the gallbladder alone. According to National Comprehensive Cancer Network guideline, individuals older than 65 years old can be categorized into elder population, thus we used 65 years old as cutoff point of age (20). We selected cancer-specific survival (CSS) as the outcomes of interest, and it was calculated from diagnosis to death or to the date of last follow-up; only GBASC-related fatalities were deemed occurrences, whereas other deaths and survivors were censored.
Due to the huge difference in clinicopathological features between early stage and advanced stage of the carcinoma, our therapeutic analysis was stratified by tumor stage. Pearson chi-squared analysis was employed to test and compare the clinical differences between patients with early-stage and advanced-stage GBASC. The Kaplan-Meier curve, the 1-, 2-, and 3-year CSS rate, and median survival were calculated using Kaplan-Meier analysis to predict the CSS of patients with GBASC with regard to different therapeutic mode. Limited to the low mortality of some cohorts in the sample, some data on median survival and 95% confidence intervals (CIs) were not available. The log-rank test was used to examine the differences between the curves.
Cox proportional hazards models were used to identify the independent prognostic variables. Univariate Cox regression was performed to reveal the potential predictors for patients with GBASC. Statistically significant variables identified by univariate Cox regression were selected for the multivariate Cox regression. Following Harrell’s rule (the number of events should be at least 10 times greater than the number of covariates), the potential predictors were included in the analysis (21). Multivariate Cox proportional hazards models were then created to evaluate independent variables with stepwise regression based on the minimum Akaike information criterion, and the hazard ratios (HRs) and 95% CIs of the independent prognostic factors associated with CSS in patients with GBASC were generally assumed. A forest map was used to visualize the efficiency of all independent variables in the analysis. Statistical analysis was performed using R software (version 4.6.3; The R Foundation of Statistical Computing, USA).
Results
Demographic and clinical characteristics
The overall sample size included 388 SEER-Medicare patients diagnosed with GBASC between the year 1975 and 2018. In all, 312 patients had died of GBASC at the time of analysis, the median follow-up time was 15.2 months, and the longest follow-up period was 165 months. Among the samples, 80 patients were classified as early stage, and 308 patients were classified as advanced stage. The majority of patients with GBASC were older patients, with a marital status of married or single, in both the early stage and advanced stage, and without significant differences between the two stages. A greater proportion of females (75.6%) and White individuals (76.3%) was found in the advanced stage when compared with the early stage (60.0% and 68.8%, respectively) (P<0.001 for both). Among the treatment options, nonradical surgery was used in almost all patients from the early stage (92.5%) and part of patients from the advanced stage (62.3%), while radical surgery was performed mainly in later-stage disease patients (23.4%; P<0.001). The remaining treatment modalities shared similar distributions between the two stages: lymph node dissection was performed in half of the patients, and chemotherapy and radiotherapy were used to treat fewer than one-eighth of the patients with GBASC, respectively. A significant difference in pathological differentiation existed between the patients of two stages: moderately differentiated carcinoma constituted half of the early-stage patients (51.3%), while poorly differentiated carcinoma was observed in most of the patients with advanced GBASC (59.7%; P<0.001; Table 1). Compared to patients with advanced GBASC, patients with early-stage GBASC (median survival: 21 vs. 6 months, P<0.001) had substantially longer overall survival (Figure 1, Table 2).
Table 1
| Patient variables | Advanced stage (n=308) | Early stage (n=80) | Overall (n=388) | P |
|---|---|---|---|---|
| Age, n (%) | 0.178 | |||
| <65 years old | 124 (40.3) | 25 (31.3) | 149 (38.4) | |
| ≥65 years old | 184 (59.7) | 55 (68.8) | 239 (61.6) | |
| Sex, n (%) | 0.008 | |||
| Female | 233 (75.6) | 48 (60.0) | 281 (72.4) | |
| Male | 75 (24.4) | 32 (40.0) | 107 (27.6) | |
| Race, n (%) | 0.004 | |||
| White | 235 (76.3) | 55 (68.8) | 290 (74.7) | |
| American Indian/Alaska Native | 4 (1.3) | 0 (0.0) | 4 (1.0) | |
| Asian or Pacific Islander | 45 (14.6) | 8 (10.0) | 53 (13.7) | |
| Black | 24 (7.8) | 17 (21.3) | 41 (10.6) | |
| Marital status, n (%) | 0.794 | |||
| Divorced | 23 (7.5) | 5 (6.3) | 28 (7.2) | |
| Married | 153 (49.7) | 43 (53.8) | 196 (50.5) | |
| Single | 132 (42.9) | 32 (40.0) | 164 (42.3) | |
| Surgery, n (%) | <0.001 | |||
| No surgery | 44 (14.3) | 0 (0.0) | 44 (11.3) | |
| Nonradical surgery | 192 (62.3) | 74 (92.5) | 266 (68.6) | |
| Radical surgery | 72 (23.4) | 6 (7.5) | 78 (20.1) | |
| Lymph node dissection, n (%) | 0.494 | |||
| 0 | 166 (53.9) | 47 (58.8) | 213 (54.9) | |
| 1–3 | 73 (23.7) | 14 (17.5) | 87 (22.4) | |
| ≥4 | 69 (22.4) | 19 (23.8) | 88 (22.7) | |
| Chemotherapy, n (%) | 0.119 | |||
| No | 164 (53.2) | 51 (63.8) | 215 (55.4) | |
| Yes | 144 (46.8) | 29 (36.3) | 173 (44.6) | |
| Radiotherapy, n (%) | 0.382 | |||
| No | 240 (77.9) | 58 (72.5) | 298 (76.8) | |
| Yes | 68 (22.1) | 22 (27.5) | 90 (23.2) | |
| Grade, n (%) | <0.001 | |||
| Well differentiated | 4 (1.3) | 8 (10.0) | 12 (3.1) | |
| Moderately differentiated | 110 (35.7) | 41 (51.3) | 151 (38.9) | |
| Poorly differentiated | 184 (59.7) | 31 (38.8) | 215 (55.4) | |
| Undifferentiated, anaplastic | 10 (3.2) | 0 (0.0) | 10 (2.6) |
GBASC, adenosquamous carcinoma of the gallbladder.
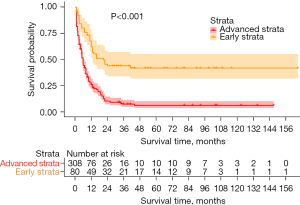
Table 2
| AJCC stage | Treatment | 1-year survival, % | 2-year survival, % | 3-year survival, % | Median survival (months) | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Early stage | Including all treatment | 62.20 | 44.00 | 41.80 | 21 | 13–NA | <0.001 |
| Advanced stage | Including all treatment | 25.90 | 10.30 | 7.30 | 6 | 5–7 | |
| Early stage | ≥4 lymph nodes dissection | 94.70 | 73.70 | 73.70 | NA | NA–NA | <0.001 |
| Early stage | 1–3 lymph nodes dissection | 69.20 | 43.30 | 43.30 | 22 | 11–NA | |
| Early stage | No lymph node dissection | 45.10 | 30.10 | 30.10 | 12 | 7–21 | |
| Early stage | Chemotherapy | 65.50 | 33.60 | 33.60 | 16 | 13–NA | 0.500 |
| Early stage | No chemotherapy | 60.40 | 51.10 | 51.10 | NA | 11–NA | |
| Early stage | Radiotherapy | 54.50 | 27.30 | 27.30 | 13 | 12–41 | 0.100 |
| Early stage | No radiotherapy | 65.70 | 51.60 | 51.60 | NA | 14–NA | |
| Advanced stage | Radical surgery | 44.50 | 19.70 | 13.80 | 11 | 9–16 | <0.001 |
| Advanced stage | Nonradical surgery | 24.70 | 9.50 | 7.00 | 6 | 5–7 | |
| Advanced stage | No surgery | 2.30 | 0.00 | 0.00 | 2 | 1–3 | |
| Advanced stage | ≥4 lymph nodes dissection | 48.20 | 24.00 | 17.40 | 12 | 9–16 | <0.001 |
| Advanced stage | 1–3 lymph nodes dissection | 31.60 | 9.50 | 7.60 | 8 | 7–11 | |
| Advanced stage | No lymph node dissection | 14.60 | 5.70 | 3.60 | 4 | 3–5 | |
| Advanced stage | Chemotherapy | 38.50 | 12.20 | 7.60 | 10 | 8–12 | <0.001 |
| Advanced stage | No chemotherapy | 14.80 | 9.90 | 7.40 | 3 | 2–4 | |
| Advanced stage | Radiotherapy | 66.30 | 25.10 | 22.80 | 17 | 13–20 | <0.001 |
| Advanced stage | No radiotherapy | 14.00 | 6.00 | 1.40 | 4 | 3–5 |
CSS, cancer-specific survival; GBASC, adenosquamous carcinoma of the gallbladder; AJCC, American Joint Committee on Cancer; CI, confidence interval; NA, not applicable.
Comparative efficacy of the different therapeutic approaches
The survival rates associated with GBASC for each of the treatment regimens at various stages of illness were calculated using Kaplan-Meier analysis. The impact of the surgery method on the early-stage patients with GBASC was not analyzed, mainly because of the uncoordinated samples between groups. The log-rank test of the Kaplan-Meier curves for the GBASC cohort at the early disease stage indicated that the lymph node dissection improved the CSS significantly only when more than 4 lymph nodes were removed. The 1-, 2-, and 3-year CSS for patients with more than 4, 1–3, and 0 lymph nodes dissected were 94.7% vs. 69.2% vs. 45.1%, 73.7% vs. 43.3% vs. 30.1%, and 73.7% vs. 43.3% vs. 30.1%, respectively (P<0.001). In the evaluation regarding the treatment efficacy of chemotherapy and radiotherapy for patients with early-stage GBASC, the log-rank test of the survival curves demonstrated no significant difference in CSS (chemotherapy: P=0.500; radiotherapy: P=0.100), indicating that chemotherapy and radiotherapy did not exert a curative effect on the early-stage group (Figure 2, Table 2).

Compared with early-staged patients, the characteristic of the relative efficacy of therapeutic approaches for patients with GBASC identified at an advanced stage altered. Radical surgery was associated with the highest CSS, followed by nonradical surgery, and then no surgery. The 1-, 2-, and 3-year CSS for patients who had radical surgery, nonradical surgery, and no surgery was 44.5% vs. 24.7% vs. 2.3%, 19.7% vs. 9.5% vs. 0%, and 13.8% vs. 7% vs. 0%, respectively (P<0.001). This suggests surgery, especially radical surgery, is a crucial treatment method for advanced patients with GBASC. Among the patients with operative treatment, GBASC-related mortality was associated with the execution of lymph node dissection but not with the number of dissected lymph nodes. The 1-, 2-, and 3-year CSS for patients with more than 4, 1–3, and 0 lymph nodes dissected was 48.2% vs. 31.6% vs. 14.6%, 24.0% vs. 9.5% vs. 5.7%, and 17.4% vs. 7.6% vs. 3.6%, respectively (P<0.001), indicating the additional removal of lymph did not confer equal benefit. The survival benefit of chemotherapy and radiotherapy on advanced patients with GBASC was also obvious: CSS was much improved, and the difference was significant. The 1-, 2-, and 3-year CSS for patients who were and were not given chemotherapy was 38.5% vs. 14.8%, 12.2% vs. 9.9%, and 7.6% vs. 7.4%, respectively (P<0.001). The 1-, 2-, and 3-year CSS for patients who were and were not given radiotherapy was 66.3% vs. 14.0%, 25.1% vs. 6.0%, and 22.8% vs. 1.4%, respectively (P<0.001). Unlike early-stage patients, terminally ill patients were sensitive to chemotherapy and radiotherapy (Figure 3, Table 2). There was a statistically significant difference in the predicted advantages of survival between any two-way comparisons that had not previously been made between the different modalities. (i.e., P<0.050 for any comparison).
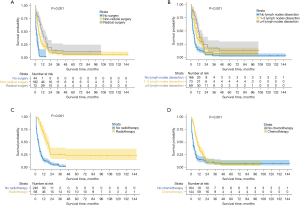
Identification of independent prognostic factors of CSS in patients with GBASC
The prognostic factors for CSS in those with GBASC were examined using univariate and multivariate Cox regression analysis. As per the univariate Cox regression, age, race, marital status, surgery, lymph nodes dissection, chemotherapy, radiotherapy, and TNM stage were associated with the CSS of patients with GBASC. Based on the statistically significant factors identified by the multivariate Cox regression analysis described above, multivariate Cox regression further confirmed 9 variables, including older age (≥65 vs. <65 years old: HR =1.41; 95% CI, 1.10–1.80; P=0.007), Asian or Pacific Islander descent (vs. White: HR =0.65; 95% CI, 0.44–0.96; P=0.031), radical surgery (vs. no surgery: HR =0.42; 95% CI, 0.26–0.67; P<0.001), nonradical surgery (vs. no surgery: HR =0.37; 95% CI, 0.25–0.54; P<0.001), 1–3 lymph nodes dissected (HR =0.66; 95% CI, 0.48–0.91; P=0.012), more than 4 lymph nodes dissected (HR =0.58; 95% CI, 0.40–0.83; P=0.003), chemotherapy (vs. no chemotherapy: HR =0.67; 95% CI, 0.51–0.88; P=0.003), radiotherapy (vs. no radiotherapy: HR =0.65; 95% CI, 0.46–0.91; P=0.011), T3–T4 stage (vs. T1–T2: HR =1.68; 95% CI, 1.27–2.21; P<0.001), M1 stage (vs. M0: HR =2.55; 95% CI, 1.94–3.34; P<0.001), and N1 stage (vs. N0: HR =1.71; 95% CI, 1.29–2.27; P<0.001) as independent predictors for the CSS of patients with GBASC (Table 3, Figure 4). Similar to Kaplan-Meier analysis, multivariate Cox regression analysis revealed that the type of treatment received by GBASC patients was a prognostic factor. The sample size, number of events and HR in each analysis were also calculated (Table 4).
Table 3
| Patient variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | |||||||
| <65 years old | Reference | Reference | |||||
| ≥65 years old | 1.42 | 0.70–1.73 | <0.001 | 1.41 | 1.10–1.80 | 0.007 | |
| Race | |||||||
| White | Reference | Reference | |||||
| American Indian/Alaska Native | 1.52 | 0.57–4.11 | 0.406 | 2.63 | 0.95–7.30 | 0.064 | |
| Asian or Pacific Islander | 0.66 | 1.53–0.46 | 0.021 | 0.65 | 0.44–0.96 | 0.031 | |
| Black | 1.26 | 0.89–1.77 | 0.187 | 1.34 | 0.93–1.92 | 0.114 | |
| Sex | |||||||
| Male | Reference | ||||||
| Female | 0.82 | 0.64–1.06 | 0.137 | ||||
| Marital status | |||||||
| Divorced | Reference | ||||||
| Single | 1.57 | 1.01–2.44 | 0.046 | ||||
| Married | 1.18 | 0.76–1.82 | 0.464 | ||||
| Surgery | |||||||
| No surgery | Reference | Reference | |||||
| Radical surgery | 0.21 | 0.14–0.31 | <0.001 | 0.42 | 0.26–0.67 | <0.001 | |
| Nonradical surgery | 0.24 | 0.17–0.34 | <0.001 | 0.37 | 0.25–0.54 | <0.001 | |
| Lymph node dissection | |||||||
| 0 | Reference | Reference | |||||
| 1–3 | 0.62 | 0.47–0.82 | <0.001 | 0.66 | 0.48–0.91 | 0.012 | |
| ≥4 | 0.38 | 0.28–0.51 | <0.001 | 0.58 | 0.40–0.83 | 0.003 | |
| Chemotherapy | |||||||
| No | Reference | Reference | |||||
| Yes | 0.70 | 0.56–0.88 | 0.002 | 0.67 | 0.51–0.88 | 0.003 | |
| Radiotherapy | |||||||
| No | Reference | Reference | |||||
| Yes | 0.45 | 0.34–0.59 | <0.001 | 0.65 | 0.46–0.91 | 0.011 | |
| Grade | |||||||
| Well differentiated | Reference | ||||||
| Moderately differentiated | 1.21 | 0.61–2.39 | 0.579 | ||||
| Poorly differentiated | 1.59 | 0.82–3.11 | 0.173 | ||||
| Undifferentiated, anaplastic | 3.29 | 1.33–8.14 | 0.010 | ||||
| T | |||||||
| T1–T2 | Reference | Reference | |||||
| T3–T4 | 1.79 | 1.40–2.29 | <0.001 | 1.68 | 1.27–2.21 | <0.001 | |
| M | |||||||
| M0 | Reference | Reference | |||||
| M1 | 3.13 | 2.46–3.99 | <0.001 | 2.55 | 1.94–3.34 | <0.001 | |
| N | |||||||
| N0 | Reference | Reference | |||||
| N1 | 1.34 | 1.06–1.70 | 0.015 | 1.71 | 1.29–2.27 | <0.001 | |
CSS, cancer-specific survival; GBASC, adenosquamous carcinoma of the gallbladder; HR, hazard ratio; CI, confidence interval.
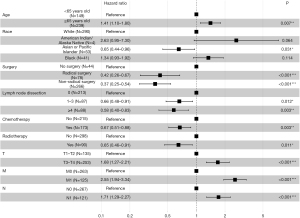
Table 4
| Patient variables | Multivariate Cox analysis | |||
|---|---|---|---|---|
| Sample size (%) | Number of events | HR (95% CI) | P | |
| Age | ||||
| <65 years old | 149 (76.51) | 114 | Reference | |
| ≥65 years old | 239 (82.95) | 198 | 1.41 (1.10–1.80) | 0.007 |
| Race | ||||
| White | 4 (100.00) | 4 | Reference | |
| American Indian/Alaska Native | 53 (64.15) | 34 | 2.63 (0.95–7.30) | 0.064 |
| Asian or Pacific Islander | 41 (92.68) | 38 | 0.65 (0.44–0.96) | 0.031 |
| Black | 290 (81.38) | 236 | 1.34 (0.93–1.92) | 0.114 |
| Surgery | ||||
| No surgery | 44 (100.00) | 44 | Reference | |
| Nonradical surgery | 266 (79.70) | 212 | 0.37 (0.25–0.54) | <0.001 |
| Radical surgery | 78 (71.79) | 56 | 0.42 (0.26–0.67) | <0.001 |
| Lymph node dissection | ||||
| 0 | 213 (88.73) | 189 | Reference | |
| 1–3 | 87 (79.31) | 69 | 0.66 (0.48–0.91) | 0.012 |
| ≥4 | 88 (61.36) | 54 | 0.58 (0.40–0.83) | 0.003 |
| Chemotherapy | ||||
| No | 215 (75.81) | 163 | Reference | |
| Yes | 173 (86.13) | 149 | 0.67 (0.51–0.88) | 0.003 |
| Radiotherapy | ||||
| No | 298 (82.89) | 247 | Reference | |
| Yes | 90 (72.22) | 65 | 0.65 (0.46–0.91) | 0.011 |
| T | ||||
| T1–T2 | 135 (69.63) | 94 | Reference | |
| T3–T4 | 253 (86.17) | 218 | 1.68 (1.27–2.21) | <0.001 |
| M | ||||
| M0 | 263 (72.24) | 190 | Reference | |
| M1 | 125 (97.60) | 122 | 2.55 (1.94–3.34) | <0.001 |
| N | ||||
| N0 | 267 (78.28) | 209 | Reference | |
| N1 | 121 (85.12) | 103 | 1.71 (1.29–2.27) | <0.001 |
HR, hazard ratio; CI, confidence interval.
Discussion
According to earlier research, GBASC is an uncommon histological variation that accounts for 2% to 10% of all GBCs (6,7). Due to the scarcity of GBASC, large clinical trials on it are difficult to conduct, resulting in little clinical evidence on its clinicopathological characteristics, survival outcomes, and treatment responses. Most related research that has focused on GBASC has been in the form of small-sample studies or data-based analyses. For instance, Murimwa et al. (13) and Akce et al. (14) characterized the clinical features of GBASC by comparing it to gallbladder adenocarcinoma (GBAC) using the National Cancer Database and SEER. According to the available literature, when compared with patients with GBAC, patients with GBASC have a larger tumor size, poorly differentiated tumors, and worse survival. These data suggests that GBASC should be explored separately rather than as a part of GBAC with no pathological subtype recognition. Previous studies identified the characteristics of GBASC relative to those of GBAC; however, there is limited research focused on the treatment response and prognostic factors; furthermore, most patients with GBASC in the previous studies were not staged for analysis, limiting the generalizability and reliability of findings (6,13,14,16). We performed the current retrospective investigation to obtain a deeper understanding of GBASC.
Our result demonstrated that most patients with GBASC were older and White, more often female, and more likely to present with advanced clinical stage. For patients in the early GBASC stage, most underwent nonradical surgery with a tumor grade of moderately or poorly differentiated, and only a few received chemotherapy or radiotherapy. Treatment modalities of patients with advanced GBASC differed from those at the early stage, with a relatively higher rate of radical surgery, chemotherapy, and radiotherapy.
Similar to previous studies, our study found that surgical excision remained the most effective treatment for curing or improving the prognosis of GBASC. Murimwa et al. (13) reported that the median survival time for patients with GBASC who received surgical removal was substantially longer than for those who did not. Additionally, Oohashi et al. (8) indicated that patients with GBASC who received radical resection were considerably more likely to survive than were those who merely underwent initial tumor excision. Similarly, Song et al. (5) reported a higher 1-year survival of patients with advanced GBASC when R0 resection was performed. Our studies also demonstrated a better survival rate in patients with advanced GBASC who underwent surgery and lymph node dissection. However, the efficacy of surgery is largely based on clinical staging and the applied surgical procedures; when considering the patients at an early stage, a similar survival benefit was found in both radical surgery and primary tumor resection. This result suggests that the range of surgery for patients with early-stage GBASC could be appropriately reduced so that patients can be guaranteed some surgical benefit with less trauma, while more radical surgery for patients with advanced GBASC can enable patients to achieve the longest survival.
As the basis of biliary cancer treatment, adjuvant systemic therapy has been problematic for patients with GBC due to a dearth of clinical trials focused on this disease, with this being worse in GBASC for its even lower incidence (2,22,23). Akce et al. (14) established a connection between increased survival with receipt of adjuvant systemic therapy in patients with GBASC. However, the lack of inclusion of nontreatment variables in the group limits the reliability of the findings. Murimwa et al. (13) found chemotherapy and chemoradiation to be independent prognostic variables for patients with GBASC, and the administration of adjuvant chemoradiation treatment was related to good overall mortality in resected patients with GBASC. Still, these two studies above did not maintain separate data for patients at different stages, and thus their results are less convincing.
The current study assessed the efficacy of systemic medication by carrying out a subgroup analysis of patients with GBASC at various stages. We found that chemotherapy and radiation therapy had no significant impact on the survival time of patients with early-stage GBASC, while advanced-stage GBASC patients had a significantly improved survival time when treated with radiotherapy or chemotherapy. Unfortunately, because SEER does not record the specifics of the systemic chemotherapy and radiotherapy that were provided, our findings are not as detailed or definitive as they could be. GBC is a disease that has no clinical trials to help clinicians make decisions about the appropriateness and type of adjunctive treatment. In the absence of clinical trials, physicians will have to make these decisions based on tumor characteristics, such as tumor differentiation, margin status, and the size or involvement of adjacent tissues.
In addition to treatment-related factors like surgery, lymph node dissection, radiation therapy, and chemotherapy, our analysis showed that patients with GBASC who were younger, of Asian or Pacific Islander descent, or with a lower AJCC stage had a better CSS. Specifically, the AJCC T stage acted as an essential factor for patients with GBASC, mainly due to the robust capabilities of the proliferation of the tumor. According to Leigh et al. (17), patients with GBASC had bigger tumors (58 vs. 28 mm), greater liver infiltration (73% vs. 37%), and a higher rate of advanced AJCC stage (73% vs. 52%) than did those with GBAC (4,8,16). In line with the aforementioned studies, we discovered that patients with GBASC had a bulky disease presentation (60 mm). According to Charbit et al. (24), the squamous carcinoma component of tumors, which develops twice as rapidly as does the AC component, is most likely responsible for the cancer’s very aggressive biological features (the doubling times are 81 and 166 days, respectively). Some scholars believe that enhanced proliferating cell nuclear antigen action in the squamous component of GBASC might contribute to the higher rate of progression as shown by bigger, locally progressed tumors; this would explain the incidence of bulky tumors and surrounding organ contacts in patients with GBASC and thus make T stage an important prognostic indicator for patients with GBASC (25).
The prognostic significance of distant metastases and lymph node involvement in GBC is well documented. However, the lymph node distribution pattern in GBASC remains unknown. Murimwa et al. (13) and Kim et al. (6) reported a much higher prevalence of lymphovascular invasion in GBASC than AC, indicating that a squamous histological element of GBC denotes a locally infiltrative disease with a higher risk of lymphatic dispersion. On the contrary, Kalayarasan et al. (16) found lower rates of nodal metastases and theorized that GBASC has a low ability to spread to other parts of the body because it enters directly through the gallbladder wall instead of through the lymphatic system. Oohashi et al. (8) reached the same conclusion. In our investigation, we discovered a low prevalence of lymph node involvement in 31% of all patients with GBASC as well as less than 50% in advanced patients with GBASC, suggesting that tumors are scattered by direct extension with fewer lymph node metastases.
In our present study, the comparison of different treatment modalities of patients with GBASC from the SEER database indicated that the differences in therapeutic effects could be significant between AJCC stages. The survival of patients with GBASC in early stages could benefit from more thorough lymph node dissection (more than 4), while advanced patients showed better survival when treated with surgery (both radical surgery and nonradical surgery), lymph node dissection during surgery, and chemotherapy and radiotherapy. Multivariate Cox regression also revealed that the aforementioned therapy modalities, as well as age, race, M stage, and N stage, were independent prognostic markers for all patients with GBASC in terms of CSS. The result provides some information for clinicians to reconsider in formulating a treatment strategy for patients with GBASC, and the prognostic factors may also help these patients receive personalized survival assessment.
Consideration should be given to the following limitations when interpreting the results of this investigation. First, the only information concerning the surgery was the procedure that was completed, and the SEER did not provide an information on the condition of the surgical margin. Aside from the type of surgery, achieving negative microscopic margins (R0 resection) was found to be the most important driver of surgical results in early GBASC. Furthermore, the lack of information on chemotherapy and radiotherapy protocols in SEER hindered our assessment of the efficacy of systematic treatment for GBASC. Moreover, the significance of neoadjuvant chemo/radiotherapy for patients with GBASC remains unclear. A clarification of the exact routine of systematic therapies, the order of surgery and systematic therapy, and the integrated treatment modalities is required to better comprehend the therapeutic response in GBASC. However, this study improves upon past case-series’ reporting for such uncommon malignancies with a larger sample size but with the tradeoff of less clinical data. Despite these limitations, our study has yielded convincing findings with implications for diagnosing and treating GBASC.
Conclusions
Intraoperative lymph node dissection may prolong the survival of patients with early-stage GBASC; surgical treatment (particularly radical surgery), lymph node dissections that are more comprehensive, radiotherapy, and chemotherapy may provide substantially improved survival benefits for patients with advanced GBASC. GBASC patient prognosis is influenced by independent risk variables, including surgery, lymph node dissection, radiation, chemotherapy, age, race, and AJCC stage.
Acknowledgments
We acknowledge the support of the Ningbo Digestive System Tumor Clinical Medicine Research Center from Ningbo Medical Centre Lihuili Hospital and of all the members who made contributions to this work.
Funding: This work was supported by the Ningbo Digestive System Tumor Clinical Medicine Research Center (No. 2019A21003) and the Ningbo Public Welfare Science & Technology Major Project (No. 2021S106).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1292/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1292/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1292/coif). All authors report that this work was supported by the Ningbo Digestive System Tumor Clinical Medicine Research Center (No. 2019A21003) and the Ningbo Public Welfare Science & Technology Major Project (No. 2021S106). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang J, Patel HK, Boakye D, et al. Worldwide distribution, associated factors, and trends of gallbladder cancer: A global country-level analysis. Cancer Lett 2021;521:238-51. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Roa JC, García P, Kapoor VK, et al. Gallbladder cancer. Nat Rev Dis Primers 2022;8:69. [Crossref] [PubMed]
- Roa JC, Tapia O, Cakir A, et al. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol 2011;24:1069-78. [Crossref] [PubMed]
- Song HW, Chen C, Shen HX, et al. Squamous/adenosquamous carcinoma of the gallbladder: Analysis of 34 cases and comparison of clinicopathologic features and surgical outcomes with adenocarcinoma. J Surg Oncol 2015;112:677-80. [Crossref] [PubMed]
- Kim WS, Jang KT, Choi DW, et al. Clinicopathologic analysis of adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol 2011;103:239-42. [Crossref] [PubMed]
- Satake T, Morizane C, Rikitake R, et al. The epidemiology of rare types of hepatobiliary and pancreatic cancer from national cancer registry. J Gastroenterol 2022;57:890-901. [Crossref] [PubMed]
- Oohashi Y, Shirai Y, Wakai T, et al. Adenosquamous carcinoma of the gallbladder warrants resection only if curative resection is feasible. Cancer 2002;94:3000-5. [Crossref] [PubMed]
- Li J, Yang ZL, Zou Q, et al. Squamous cell/adenosquamous carcinomas and adenocarcinomas of the gallbladder: an immunohistochemistry study of prognostic markers. Pathol Oncol Res 2014;20:285-92. [Crossref] [PubMed]
- Yang Z, Yang Z, Zou Q, et al. A comparative study of clinicopathological significance, FGFBP1, and WISP-2 expression between squamous cell/adenosquamous carcinomas and adenocarcinoma of the gallbladder. Int J Clin Oncol 2014;19:325-35. [Crossref] [PubMed]
- Yuan Y, Yang ZL, Miao XY, et al. EphB1 and Ephrin-B, new potential biomarkers for squamous cell/adenosquamous carcinomas and adenocarcinomas of the gallbladder. Asian Pac J Cancer Prev 2014;15:1441-6. [Crossref] [PubMed]
- Zhang W, Zhang J, Liang X, et al. Research advances and treatment perspectives of pancreatic adenosquamous carcinoma. Cell Oncol (Dordr) 2023;46:1-15. [Crossref] [PubMed]
- Murimwa G, Hester C, Mansour JC, et al. Comparative Outcomes of Adenosquamous Carcinoma of the Gallbladder: an Analysis of the National Cancer Database. J Gastrointest Surg 2021;25:1815-27. [Crossref] [PubMed]
- Akce M, Zakka K, Penley M, et al. Clinicopathological features and survival outcomes of rare histologic variants of gallbladder cancer. J Surg Oncol 2020;121:294-302. [Crossref] [PubMed]
- Samuel S, Mukherjee S, Ammannagari N, et al. Clinicopathological characteristics and outcomes of rare histologic subtypes of gallbladder cancer over two decades: A population-based study. PLoS One 2018;13:e0198809. [Crossref] [PubMed]
- Kalayarasan R, Javed A, Sakhuja P, et al. Squamous variant of gallbladder cancer: is it different from adenocarcinoma? Am J Surg 2013;206:380-5. [Crossref] [PubMed]
- Leigh N, Solomon D, Pletcher E, et al. Adeno-squamous and squamous cell carcinoma of the gallbladder: The importance of histology in surgical management. Am J Surg 2020;220:1242-8. [Crossref] [PubMed]
- Li J, Yang ZL, Ren X, et al. ILK and PRDX1 are prognostic markers in squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Tumour Biol 2013;34:359-68. [Crossref] [PubMed]
- Liu L, Yang ZL, Wang C, et al. The Expression of Notch 1 and Notch 3 in Gallbladder Cancer and Their Clinicopathological Significance. Pathol Oncol Res 2016;22:483-92. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abbott DE, et al. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw 2019;17:302-10. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Sutherland M, Ahmed O, Zaidi A, et al. Current progress in systemic therapy for biliary tract cancers. J Hepatobiliary Pancreat Sci 2022;29:1094-107. [Crossref] [PubMed]
- Hu ZI, Lim KH. Evolving Paradigms in the Systemic Treatment of Advanced Gallbladder Cancer: Updates in Year 2022. Cancers (Basel) 2022;14:1249. [Crossref] [PubMed]
- Charbit A, Malaise EP, Tubiana M. Relation between the pathological nature and the growth rate of human tumors. Eur J Cancer (1965) 1971;7:307-15. [Crossref] [PubMed]
- Sapuppo E, Brunetti O, Tessitore D, et al. Rare histotypes of epithelial biliary tract tumors: A literature review. Crit Rev Oncol Hematol 2023;181:103892. [Crossref] [PubMed]


