
Using a combination of fruquintinib, raltitrexed, and S-1 as a third-line treatment for metastatic colorectal cancer with co-existence of Hodgkin lymphoma: a case report
Highlight box
Key findings
• The case was characterized by the simultaneous existence of mCRC and Hodgkin lymphoma. The findings suggest that the combination of fruquintinib, raltitrexed, and S-1 might be clinically effective as late-line therapy for mCRC.
What is known and what is new?
• Patients with mCRC beyond second line treatment have a poor prognosis. The FRESCO trial prompts fuquinitinib as a third-line treatment in advanced colorectal cancer. A phase II study in our center reported the efficacy and safety of S-1 plus raltitrexed for the treatment of chemo-refractory mCRC.
• The combination of the three agents has not been reported, and the case demonstrated the efficacy and safety of the third-line therapy with fruquintinib, raltitrexed, and S-1 in mCRC.
What is the implication, and what should change now?
• This case provides new insight into third-line treatment options for mCRC. Future studies with large sample size are needed to validate these discoveries.
Introduction
Patients with metastatic colorectal cancer (mCRC) have a poor prognosis and the 5-year overall survival for stage IV disease is a dismal 11% (1). The classical first- and second-line therapies for mCRC are based on the FOLFOX [folinic acid, 5-fluorouracil (5-FU), and oxaliplatin] and FOLFIRI (folinic acid, 5-FU, and irinotecan) regimens (2,3). However, many patients on these standard therapies experience disease progression. Fruquintinib, regorafenib, trifluridine/tipiracil (TAS-102) are now available as third-line therapies for mCRC following the failure of first- and second-line chemotherapy regimens (2-4). Randomized controlled trial (RCT) comparing the efficacy and safety of fruquintinib, regorafenib and TAS-102 as third-line therapy in mCRC are lacking. Indirect comparison suggested that the three agents had similar OS but that fruquintinib was superior in terms of PFS and DCR compared with that of TAS-102 (4-6). However, Patients with mCRC beyond second line treatment have a poor prognosis. More third-line treatment regimens are badly needed.
Most patients with mCRC receive long-term treatment with 5-FU and/or 5-FU analogs. An important mechanism underlying resistance to 5-FU, especially secondary resistance, involves the up-regulation of dihydropyrimidine dehydrogenase (DPD) and thymidylate synthase (TS), and inhibition of these enzymes can reverse resistance to 5-FU (7,8). S-1 consists of tegafur, 5-chloro-2-4-dihydroxypyridine (which inhibits DPD), and oteracil potassium, while raltitrexed is a TS-specific inhibitor. A phase II study reported that S-1 plus raltitrexed was safe and effective for the treatment of chemo-refractory mCRC (9). Another phase II study demonstrated that third-line therapy with S-1, raltitrexed, and bevacizumab achieved good efficacy in patients with mCRC (10). A small sample retrospective study also suggested low-dose apatinib plus S-1 was a potential alternative regimen for the treatment of refractory mCRC with tolerable and controlled toxicity (11). These findings strongly suggest that late-line treatment with a combination of chemotherapeutic and antiangiogenic agents have beneficial effects in patients with mCRC.
Fruquintinib is a vascular endothelial growth factor receptor (VEGFR) inhibitor that highly selectively binds to and inhibits VEGFR-1, -2, and -3. The FRESCO study demonstrated the beneficial effects of fruquintinib monotherapy as a third-line treatment for mCRC (12). Fruquintinib monotherapy has already been approved by the Center for Drug Evaluation of China as third-line chemotherapeutic drug for mCRC.
This case report presents a patient with mCRC who developed Hodgkin lymphoma as a second malignancy, requiring careful cooperation between members of a multidisciplinary team to optimize the treatment of both cancers. Notably, following the failure of the mFOLFOX6 and FOLFIRI regimens, the patient responded well to third-line chemotherapy using fruquintinib, raltitrexed, and S-1. This case raises the possibility that regimens based on a combination of fruquintinib, raltitrexed, and S-1 could be used as late-line therapies for mCRC. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-39/rc).
Case presentation
A 54-year-old male patient was admitted to West China Hospital of Sichuan University due to hematochezia in June 2017. The timeline is shown in Figure 1. A colonoscopy showed a neoplasm between the rectum and sigmoid colon. Surgical resection, colorectal anastomosis, and enterolysis were performed in June 2017. Pathological evaluation of tumor specimens revealed the presence of adenocarcinoma with the wild-type RAS/RAF and without the PIK3CA mutation. The pathological stage was determined to be pT3pN1apM0. Immunohistochemistry revealed positive expression of MLH1, MSH2, MSH6, PMS2 and CK20 but not CDX, and the Ki67 expression was 45%. Therefore, the mFOLFOX6 regimen (oxaliplatin 85 mg/m2 i.v., leucovorin 400 mg/m2 i.v., and 5-FU 400 mg/m2 i.v. bolus on day 1 followed by 5-FU 2,400 mg/m2 i.v. continuous infusion over 46–48 hours) was administered as adjuvant therapy after surgery without SAEs.
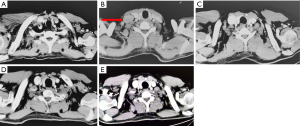
In May 2018, the patient reported dizziness, weakness, exhaustion, and fever without an evident cause. Positron emission tomography (PET) and computed tomography (CT) scanning revealed invasion of the right cervical and right supraclavicular lymph nodes by lymphoma (Figure 2). PET/CT scans also revealed lesions with increased glucose metabolism in the right lobe of the liver, which suggested hepatic metastasis of the colorectal cancer (CRC) (Figure 3). A biopsy showed hyperplasia of lymphoid tissues. Immunohistochemistry demonstrated large cells with positive expression of PAX-5, MUM-1, CD30, CD15, EBV, CXCL-13, and CD4 as well as a Ki-67 expression level of 50%. In situ hybridization detected EBER1/2. T cell receptor (TCR) gene rearrangement testing showed no clonal amplification of the TCRγ gene. The patient was diagnosed with classical Hodgkin lymphoma of mixed cellularity.
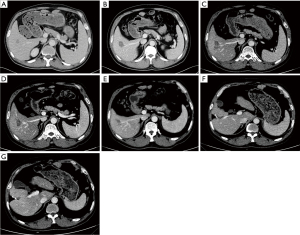
Following careful review and discussion of the case by the multidisciplinary team, the decision was made to discontinue the chemotherapy for CRC and administer 4 cycles of anti-lymphoma chemotherapy based on the ABVD regimen (doxorubicin 25 mg/m2 i.v. over 5–10 minutes, bleomycin 10,000 IU/m2 i.v. over 5–10 minutes, vinblastine 6 mg/m2 i.v. over 5–10 minutes, and dacarbazine 375 mg/m2 i.v. over 60 minutes on days 1 and 15). The lymphoma lesion showed a complete response after treatment. Re-examination by PET and CT in November 2018 showed substantial enlargement of the low-density shadow in the right liver lobe. Percutaneous liver biopsy plus radiofrequency ablation were performed as the lesion was solitary and had a diameter of less than or equal to 3 cm, and the pathological examination suggested adenocarcinoma. Taking into account the patient’s medical history, the patient was diagnosed with liver metastasis of intestinal-type adenocarcinoma (stage IV).
The patient developed liver metastasis during the first-line treatment with a PFS of 11 months. Second-line therapy with 4 cycles of the FOLFIRI regimen (irinotecan 180 mg/m2 i.v., leucovorin 200 mg/m2 i.v. and 5-FU 400 mg/m2 i.v. bolus on day 1 followed by 5-FU 600 mg/m2 i.v. continuous infusion over 22 hours) was started in January 2019. Subsequently, CT scans of the thorax and abdomen revealed new nodular lesions in the posterior segment of the apex of the superior lobe of the left lung, as well as enlargement of the lesion in the liver (September 2019; Figure 3). These findings indicated disease progression after 8 months of second-line treatments. Therefore, third-line therapy was initiated using 4-week cycles of raltitrexed 4 mg i.v. on day 1, S-1 75 mg PO on days 1–14, and fruquintinib 5 mg PO on days 1–21. Enhanced CT of the thorax and abdomen in November 2019 showed a partial response (PR) of the liver lesions to therapy (Figure 3). Upper abdominal CT in November 2020 demonstrated sporadic nodules and patchy shadows with slightly low density in the lower segment of the right liver lobe, and the margins of some lesions showed enhancement (Figure 3).
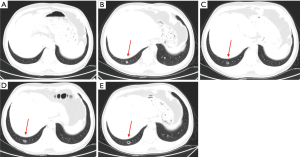
The patient now met the indications for surgery; therefore, a right hepatectomy, enterolysis, and repair of the portal vein, inferior vena cava, and small intestine were performed on November 5, 2020. Abdominal CT in January 2021 showed no evidence of tumor recurrence or further metastasis (Figure 3). Thoracic CT demonstrated a 0.7 cm diameter nodule at the posterior basal segment of the inferior lobe of the right lung (Figure 4). Therapy with raltitrexed, S-1, and fruquintinib was continued from January 2021. CT performed in April 2021 revealed cavitation of the lesion in the right lower lung, no change in the lesions in the left lung, and no recurrence of the liver lesion or lymphoma (Figure 4). In August 2021, CT showed that cavitation of the lesion in the right lower lung was enlarged (Figure 4). Furthermore, the carcinoembryonic antigen level fell from 140 to 1.86 ng/mL following therapy with these agents. The patient developed mild oral ulceration during treatment with raltitrexed, S-1, and fruquintinib, which improved after discontinuation of fruquintinib for 3 days. In November 2021, CT images showing the lymphoma, liver, and lungs revealed stable disease (Figure 4). Thus, raltitrexed was discontinued, and treatment of S-1 and fruquintinib was maintained. In April 2022, the patient was followed by a phone interview and reported grade 2 hypertension and a hand-foot skin reaction, which could be tolerated without a dose adjustment or interruption. The treatment is still responding until the last follow-up (December 2022) with a PFS of 38 months and the OS not yet reached.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case report presents a patient with chemo-refractory mCRC who also developed Hodgkin lymphoma, necessitating careful management by a multidisciplinary team. Notably, the patient achieved a PR that allowed resection of the metastatic lesion in the liver as well as a relatively long progression-free survival (PFS) of 38 months after third-line therapy with fruquintinib, raltitrexed, and S-1. The overall survival (OS) has not yet reached. In addition, there were no SAEs during treatment with this drug combination.
A previous study demonstrated the efficacy and safety of S-1 plus raltitrexed in the treatment of chemo-refractory mCRC, with an objective response rate of 13.9%, a disease control rate of 58.1%, a median PFS of 107 days [95% confidence interval (CI): 96.3–117.7 days], and overall survival (OS) of 373 days (95% CI: 226.2–519.8 days) (9). Another investigation reported that third-line treatment with S-1, raltitrexed, and bevacizumab achieved a median PFS of 110 days (95% CI: 65.0–155.0 days) and an OS of 367 days (95% CI: 310.4–420.6 days) in patients with mCRC (10). The above findings suggest that treatments based on chemotherapeutic agents and targeted antiangiogenic drugs can potentially be late-line therapies for mCRC.
Systemic chemotherapy can downregulate VEGF-A and VEGF-C (13). The currently used VEGF inhibitors, such as bevacizumab, inhibit the VEGF-A/VEGFR pathway and angiogenesis. Several phase II clinical trials have supported the combined use of an antiepidermal growth factor receptor agent and an antiangiogenic drug as a late-line treatment for mCRC, including the BOND-2 trial (cetuximab, bevacizumab and irinotecan) (14) and 2 studies by Chen et al. published in 2019 (S-1 and raltitrexed) (9) and 2021 (S-1, raltitrexed and bevacizumab) (10). However, the phase III PACCE trial (oxaliplatin or irinotecan plus bevacizumab with or without panitumumab) showed no benefit of the combination (15). Further, the phase III CAIRO2 trial (bevacizumab and capecitabine/oxaliplatin with or without cetuximab) suggested a shortening of PFS in patients who received bevacizumab and cetuximab (16). Hence, the response of mCRC to late-line treatment likely depends on the combination of agents used, highlighting the need for additional research to establish optimal drug combinations.
The effects of VEGF inhibitors on VEGF-C-induced lymphangiogenesis are limited. Fruquintinib inhibits VEGFR-1, -2, and -3 and suppresses all VEGFR-dependent pathways, including tumor angiogenesis and lymphangiogenesis (17). Therefore, we speculated that the combination of fruquintinib, S-1, and raltitrexed might exert strong antitumor effects. The patient described in this case report exhibited a PR to treatment with this combination of drugs that allowed him to meet the indications for surgery and achieve a long PFS. The potential benefits of fruquintinib-based combination therapies are supported by another case report describing a patient with mCRC who was successfully treated with cetuximab and fruquintinib (18). Nevertheless, the patient in the present study developed lung metastases during treatment. We speculate that pulmonary metastasis may have occurred following the temporary discontinuation of the fruquintinib-based therapy from October 2019 to February 2020 due to liver surgery.
The patient described here was diagnosed with Hodgkin lymphoma in addition to mCRC. Reports of patients with simultaneous solid and hematologic primary malignancies are uncommon. Diagnosis of the patient’s malignancies, evaluation of the patient’s clinical condition, and selection of the therapeutic regimens after weighing up the possible advantages and disadvantages necessitated close cooperation and detailed discussions between members of a multidisciplinary team that included physicians from the Hematology Department. The lymphoma was considered a treatment priority for this patient because the tumor burden and the Ki67 index were high. Furthermore, it was considered that treatment of the lymphoma would control its clinical manifestations, such as dizziness, exhaustion, and fever. In addition, chemotherapy can potentially cure early-stage lymphoma. Therefore, the multidisciplinary team made the decision to discontinue the chemotherapy for CRC and administer 4 cycles of antilymphoma chemotherapy based on the ABVD regimen. This approach successfully controlled the lymphoma without preventing subsequent treatment of the mCRC from achieving good efficacy.
Conclusions
In conclusion, this report describes a rare case of a patient with mCRC who also developed Hodgkin lymphoma. Successful management of both primary malignancies required close cooperation between a multidisciplinary team. Importantly, third-line treatment of the mCRC with a combination of fruquintinib, raltitrexed, and S1 achieved a PR that enabled the patient to undergo surgery for liver metastasis. The findings suggest that regimens based on this drug combination might be clinically effective as late-line therapy for mCRC, although this will need to be confirmed by clinical trials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-39/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-39/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Crutcher M, Waldman S. Biomarkers in the development of individualized treatment regimens for colorectal cancer. Front Med (Lausanne) 2022;9:1062423. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 2.2022: National Comprehensive Cancer Network; 2022.
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:10-32. [Crossref] [PubMed]
- Xu X, Yu Y, Liu M, et al. Efficacy and safety of regorafenib and fruquintinib as third-line treatment for colorectal cancer: a narrative review. Transl Cancer Res 2022;11:276-87. [Crossref] [PubMed]
- Zhang Q, Wang Q, Wang X, et al. Regorafenib, TAS-102, or fruquintinib for metastatic colorectal cancer: any difference in randomized trials? Int J Colorectal Dis 2020;35:295-306.
- Chen J, Wang J, Lin H, et al. Comparison of Regorafenib, Fruquintinib, and TAS-102 in Previously Treated Patients with Metastatic Colorectal Cancer: A Systematic Review and Network Meta-Analysis of Five Clinical Trials. Med Sci Monit 2019;25:9179-91. [Crossref] [PubMed]
- Li LH, Dong H, Zhao F, et al. The upregulation of dihydropyrimidine dehydrogenase in liver is involved in acquired resistance to 5-fluorouracil. Eur J Cancer 2013;49:1752-60. [Crossref] [PubMed]
- Subbarayan PR, Sarkar M, Nelson G, et al. Chronic exposure of colorectal cancer cells in culture to fluoropyrimidine analogs induces thymidylate synthase and suppresses p53. A molecular explanation for the mechanism of 5-FU resistance. Anticancer Res 2010;30:1149-56.
- Chen Y, Wu J, Cheng K, et al. S-1 plus Raltitrexed for Refractory Metastatic Colorectal Cancer: A Phase II Trial. Oncologist 2019;24:591-e165. [Crossref] [PubMed]
- Chen Y, Zhou YW, Cheng K, et al. Bevacizumab Combined with S-1 and Raltitrexed for Patients with Metastatic Colorectal Cancer Refractory to Standard Therapies: A Phase II Study. Oncologist 2021;26:e1320-6. [Crossref] [PubMed]
- Dai Y, Sun L, Zhuang L, et al. Efficacy and safety of low-dose apatinib plus S-1 versus regorafenib and fruquintinib for refractory metastatic colorectal cancer: a retrospective cohort study. J Gastrointest Oncol 2022;13:722-31. [Crossref] [PubMed]
- Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018;319:2486-96. [Crossref] [PubMed]
- Tampellini M, Sonetto C, Scagliotti GV. Novel anti-angiogenic therapeutic strategies in colorectal cancer. Expert Opin Investig Drugs 2016;25:507-20. [Crossref] [PubMed]
- Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol 2007;25:4557-61. [Crossref] [PubMed]
- Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009;27:672-80. [Crossref] [PubMed]
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563-72. [Crossref] [PubMed]
- Zhang Y, Zou JY, Wang Z, et al. Fruquintinib: a novel antivascular endothelial growth factor receptor tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. Cancer Manag Res 2019;11:7787-803. [Crossref] [PubMed]
- Li Y, Chen X, Li W, et al. Combination of Anti-EGFR and Anti-VEGF Drugs for the Treatment of Previously Treated Metastatic Colorectal Cancer: A Case Report and Literature Review. Front Oncol 2021;11:684309. [Crossref] [PubMed]


