
Drug-eluting bead transarterial chemoembolization could improve the hepatic hemodynamics of patients with unresectable hepatocellular carcinoma: a retrospective cohort study
Highlight box
Key findings
• DEB-TACE could improve the hepatic hemodynamics in HCC patients with liver cirrhosis, especially those with CSPH, which is an important prognostic factor for survival.
What is known and what is new?
• Conventional TACE is widely used for patients with unresectable HCC, which might influence the hepatic hemodynamics. As a newly developed local regional therapy, DEB-TACE showed superior treatment efficacy and safety to conventional TACE.
• DEB-TACE treatment could beneficially influence hepatic hemodynamics in HCC patients and decrease HVPG levels, especially for those with CSPH; moreover, CSPH is a negative prognostic factor for such patients.
What is the implication, and what should change now?
• Considering that DEB-TACE can control tumor progression but also decrease portal hypertension and relieve underlying liver disease, it might be more suitable for the treatment of HCC patients with CSPH than conventional TACE.
Introduction
Primary liver cancer is currently the second leading cause of cancer-related death worldwide, among which hepatocellular carcinoma (HCC) accounts for >90% (1,2). The Barcelona Clinic Liver Cancer (BCLC) staging system is widely used for prognostic staging and treatment recommendations in HCC (3,4). According to this staging system, transarterial chemoembolization (TACE) is the first-line treatment option for patients with intermediate HCC (5). However, the use of TACE is often beyond this recommendation in current practice, and it might also benefit both early-stage patients unsuitable for curative treatments and advanced-stage patients with preserved liver function and limited tumor burden (6-10).
According to previous studies, conventional TACE (cTACE) treatment outcomes achieved a median survival of 19.4 months for general patients with unresectable HCC in clinical practice (11,12). Drug-eluting bead TACE (DEB-TACE) is a newly developed local regional therapy for improving the efficacy and safety of cTACE and is now universally used to treat patients with unresectable HCC (13,14). Diverse studies have shown DEB-TACE to be associated with favorable treatment responses, prolonged survival, and at least similar safety and less common adverse events than cTACE (15-17).
Different from other types of solid malignancies, the prognosis of HCC patients is influenced not only by tumor itself but also by the underlying liver diseases; therefore, prognostic factors including tumor burden, liver function and performance status were closely correlated with the survival of HCC patients (18,19). Hepatic hemodynamics of patients with cirrhosis were usually characterized of portal hypertension, which was both relevant with the decompensated events and patient prognosis. Particularly, clinically significant portal hypertension (CSPH) is highly correlated with the long-term survival of HCC patients. Several studies have demonstrated that cTACE seems to have transient effects on hepatic hemodynamics, with a decrease in hepatic arterial blood flow, an increase in portal blood flow, and a long-term effect due to an increase in the hepatic venous pressure gradient (HVPG) (20-24). However, such relevant changes following DEB-TACE therapy for HCC patients have rarely been reported. Consequently, in the present study we aimed to investigate the effects of DEB-TACE on hepatic hemodynamics measured by HVPG, as well as its prognostic factors including CSPH in patients with unresectable HCC. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-76/rc).
Methods
From April 2018 to September 2020, consecutive patients with cirrhosis and HCC who were treated with DEB-TACE were recruited in this retrospective cohort study. The diagnosis of HCC was mainly based on typical imaging findings in computed tomography (CT) or magnetic resonance imaging assessment with arterial phase hyperenhancement and portal venous or delayed phase washout; when necessary, pathological examination was used for diagnostic confirmation (5). Patients meeting the following characteristics were excluded from this cohort: younger than 18 or older than 75 years, severely decompensated liver function (refractory ascites, jaundice or hepatic encephalopathy), huge tumor burden (maximum diameter of the largest tumor >10 cm, diffused tumor, main portal vein tumor thrombosis (PVTT) or extrahepatic spread), and poor performance status with Eastern Cooperative Oncology Group (ECOG) score >2. Eventually, a total of 24 eligible patients were included in this study. Laboratory examinations including routine blood test, liver and kidney function, and tumor markers were performed before 1–2 days of treatment; imaging assessments were judged by two different investigators, and the final decision was rendered based on discussions when disagreements arose.
DEB-TACE was performed in all cases. Briefly, the Seldinger technique was used to intubate the femoral artery via percutaneous puncture, and then the location, size, number and supply arteries of the tumors were identified by digital subtraction angiography. Next, a hybrid emulsion of CalliSpheres® microspheres (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) loaded with doxorubicin (60–80 mg) was infused into the supply arteries, and all distal branches were embolized as much as possible. During follow-up, laboratory examination and imaging assessment were performed 4–6 weeks after each procedure. For patients with preserved liver function, repeated TACE sessions were implemented upon confirmation of viable tumor or local and/or distant intrahepatic recurrences. During the study period, none participant was lost to follow-up. Overall survival (OS) was defined as the time from DEB-TACE treatment until death or last follow-up.
According to previously reported operating procedures, HVPG measurement was performed for every patient before or after TACE therapy. Briefly, a balloon occlusion catheter was introduced through the inferior vena cava into a large liver vein. Wedged hepatic vein pressure (WHVP) and free hepatic vein pressure (FHVP) were separately measured three times, and the difference in their mean values was defined as the HVPG. During the standardized determination of HVPG, contrast medium (5 mL) was injected before the WHPG measurement to exclude the selected hepatic vein without collateral branches. In addition, those with HVPG ≥10 mmHg were defined as patients with CSPH. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by Institutional Ethics Board of The Affiliated Yantai Yuhuangding Hospital of Qingdao University (No. Yanyuyi 2018-312). Individual consent for this retrospective analysis was waived.
Statistical analysis
Baseline categorical variables are described by frequencies and percentages and compared using the chi-square test or Fisher’s precision probability test among patients with and without CSPH; continuous data are described as the median plus interquartile range (IQR) and compared by t-test or the Rand sum test. Changes of portal hemodynamics were indicated by the measurement of WHVP, FHVP and HVPG before and after DEB-TACE; through comparing means and medians of these values, the influence of DEB-TACE on portal hemodynamics was clearly illustrated. Survival analysis was carried out using the Kaplan-Meier method, and the differences between curves were assessed by the log-rank test. The Cox proportional hazard model was used to assess the prognostic value of the variables, especially for CSPH which represented the portal hemodynamics of patients. Considering that CSPH was closely correlated with liver-function which was also the significant prognostic factors, the prognostic abilities of CSPH were adjusted by liver function and other significant factors in multivariate analysis. Statistical analysis was performed using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA), and a two-sided P value <0.05 was considered significant.
Results
The baseline demographic and clinical characteristics of the patient population are summarized in Table 1. Among the 24 patients with a median age of 58.0 (IQR 54.4–68.0) years, 16 patients (66.7%) were male; hepatitis B virus (HBV) was the most common underlying cause of liver disease (20, 83.3%). Altogether, 13 patients (54.2%) were in Child-Pugh A class, and 11 patients (45.8%) were in Child-Pugh B class; the median Model for End-Stage Liver Disease (MELD) score of the entire patient cohort was 9.5 (IQR 9–12) points. The included patients had no or mild cancer-related symptoms, with ECOG performance status scores of 0 (18, 75.0%) or 1 (6, 25.0%). There were 10 patients with PVTT and 18 patients with α-fetoprotein (AFP) levels >400 ng/mL. In addition, the median WHVP and FHVP were 15.7 (IQR 11.5–24.3) mmHg and 8.85 (IQR 7.78–10.7) mmHg, respectively; accordingly, the median HVPG level reached 6.15 (IQR 3.97–11.3) mmHg. Finally, 7 (29.2%) patients with HVPG ≥10 mmHg were defined as CSPH; otherwise, they were defined as non-CSPH (17, 70.8%). Comparing the baseline characteristics of the non-CSPH and CSPH patients revealed that the CSPH patients were older, had higher ECOG, Child-Pugh and MELD scores; higher levels of total bilirubin (TBIL), aspartate aminotransferase (AST) and serum creatinine (Cr), but lower body mass index (BMI), albumin (ALB) and platelets, as well as a greater frequency of non-HBV etiology, ascites and esophageal-gastro varices (EGV). Additionally, compared with the non-CSPH patients, those with CSPH had higher WHVP, FHVP and HVPG.
Table 1
| Variable | Total | Non-CSPH | CSPH |
|---|---|---|---|
| Patients, n | 24 | 17 | 7 |
| Sex, male/female, n (%) | 16 (66.7)/8 (33.3) | 10 (58.8)/7 (41.2) | 6 (85.7)/1 (14.3) |
| Age, years, median [IQR]* | 58.0 [54.5, 68.0] | 58.0 [52.5, 61.0] | 69.0 [56.0, 72.0] |
| Etiology, HBV/other, n (%)* | 20 (83.3)/4 (16.7) | 16 (94.1)/1 (5.9) | 4 (57.1)/3 (42.9) |
| Height, m, median [IQR] | 1.71 [1.63, 1.77] | 1.70 [1.63, 1.78] | 1.71 [1.68, 1.72] |
| Weight, kg, median [IQR] | 62 [56.8, 67.8] | 62.0 [59.0, 74.0] | 56.0 [55.0, 62.0] |
| BMI, kg/m2, median [IQR]* | 21.6 [20.2, 22.6] | 22.5 [21.0, 23.1] | 19.8 [18.9, 21.2] |
| PVTT, no/yes, n (%) | 14 (58.3)/10 (41.7) | 10 (58.8)/7 (41.2) | 4 (57.1)/3 (42.9) |
| Ascites, no/yes, n (%)* | 19 (79.2)/5 (20.8) | 17 (100.0)/0 (0.0) | 2 (28.6)/5 (71.4) |
| ECOG performance status, 0/1, n (%)* | 18 (75.0)/6 (25.0) | 16 (94.1)/1 (15.9) | 2 (28.6)/5 (71.4) |
| EGV, no/yes, n (%)* | 14 (58.3)/10 (41.7) | 11 (64.7)/6 (35.3) | 2 (28.6)/5 (71.4) |
| Child-Pugh class, A/B, n (%)* | 13 (54.2)/11 (45.8) | 13 (76.5)/4 (23.5) | 0 (0.0)/7 (100.0) |
| Child-Pugh score, median [IQR]* | 6 [5, 8] | 5 [5, 7] | 9 [7, 9] |
| MELD score, median [IQR]* | 9.5 [9, 12] | 9 [7, 11] | 12 [11, 14] |
| ALT, U/L, median [IQR] | 41.5 [35, 46.5] | 41.0 [32.0, 49.5] | 44.0 [35.0, 44.0] |
| AST, U/L, median [IQR]* | 48 [24, 76] | 28.0 [24.0, 50.0] | 76.0 [76.0, 87.0] |
| ALB, g/L, median [IQR]* | 35.2 [30.0, 40.2] | 36.0 [34.9, 42.1] | 27.2 [26.3, 27.2] |
| TBIL, μmol/L, median [IQR]* | 20.9 [18.8, 26.8] | 19.6 [18.8, 25.5] | 28.6 [19.1, 29.3] |
| Cr, μmol/L, median [IQR]* | 48 [43.8, 54.3] | 44.0 [42.0, 50.0] | 60.0 [51.0, 60.0] |
| BUN, mmol/L, median [IQR] | 4.74 [3.68, 6.09] | 3.8 [3.5, 6.0] | 4.9 [4.6, 6.3] |
| INR, median [IQR] | 1.11 [1.05, 1.30] | 1.08 [1.03, 1.13] | 1.32 [1.30, 1.41] |
| Platelets, ×109/L* | 88.5 [67.8, 166.3] | 132 [77, 182] | 69 [45, 88] |
| AFP, ≤400/>400 ng/mL, n (%) | 6 (25.0)/18 (75.0) | 4 (23.5)/13 (76.5) | 2 (28.6)/5 (71.4) |
| WHVP, mmHg, median [IQR]* | 15.7 [11.5, 24.3] | 14.0 [11.0, 15.8] | 29.0 [24.3, 29.0] |
| FHVP, mmHg, median [IQR]* | 8.85 [7.78, 10.7] | 8.30 [7.50, 9.00] | 13.0 [10.7, 16.3] |
| HVPG, mmHg, median [IQR]* | 6.15 [3.97, 11.3] | 6.00 [3.03, 6.50] | 12.0 [11.3, 18.3] |
*, variables with significant difference between patients with and without CSPH. CSPH, clinically significant portal hypertension; IQR, interquartile range; HBV, hepatitis B virus; BMI, body mass index; PVTT, portal vein tumor thrombosis; ECOG, Eastern Cooperative Oncology Group; EGV, esophageal-gastro varices; MELD, Model for End-Stage Liver Disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBIL, total bilirubin; Cr, serum creatinine; BUN, blood urea nitrogen; INR, international normalized ratio; AFP, alpha-fetoprotein; WHVP, wedged hepatic vein pressure; FHVP, free hepatic vein pressure; HVPG, hepatic venous pressure gradient.
During a median follow-up of 9.8 months, 17 patients died, with a median OS of 10.0 months for the whole cohort. The prognostic abilities for baseline variables, including sex, age, etiology, PVTT, ascites, ECOG score, EGV, Child-Pugh class, MELD score, alanine aminotransferase (ALT), AST, ALB and AFP, were analyzed (Table 2). Kapan-Meier survival curve analysis demonstrated that age >60 years (log-rank P=0.023), ascites (log-rank P<0.001), ECOG score of 1 (log-rank P<0.001), Child-Pugh B class (log-rank P<0.001), MELD score >10 (log-rank P<0.001), and ALB <35 g/L (log-rank P=0.002) were significant prognostic factors for decreased OS. Similarly, Cox regression analysis also revealed that age >60 years [hazard ratio (HR) 2.97, 95% confidence interval (CI): 1.05–8.38, P=0.040], ascites (HR 11.6, 95% CI: 2.73–49.8, P=0.001), ECOG score of 1 (HR 5.42, 95% CI: 1.77–16.6, P=0.003), Child-Pugh B class (HR 6.22, 95% CI: 2.02–19.1, P=0.001), MELD score >10 (HR 6.38, 95% CI: 1.95–20.8, P=0.002), and ALB <35 g/L (HR 5.37, 95% CI: 1.57–18.3, P=0.007) were statistically correlated with OS.
Table 2
| Variable | Kaplan-Meier curve analysis | Cox regression analysis | ||||
|---|---|---|---|---|---|---|
| No. of patients | Median OS (months) | Log-rank P value | HR (95% CI) | P value | ||
| Sex, male/female | 16/8 | 8/15 | 0.202 | 0.51 (0.17–1.58) | 0.243 | |
| Age >60 years, no/yes | 15/9 | 15/7 | 0.023 | 2.97 (1.05–8.38) | 0.040 | |
| HBV infection, no/yes | 4/20 | 6/10 | 0.315 | 1.74 (0.48–6.29) | 0.396 | |
| PVTT, no/yes | 14/10 | 15/9 | 0.261 | 0.60 (0.23–1.58) | 0.301 | |
| Ascites, no/yes | 19/5 | 15/6 | <0.001 | 11.6 (2.73–49.8) | 0.001 | |
| ECOG score, 0/1 | 18/6 | 15/6 | <0.001 | 5.42 (1.77–16.6) | 0.003 | |
| EGV, no/yes | 11/10 | 11/8 | 0.905 | 0.94 (0.32–2.75) | 0.910 | |
| Child-Pugh class, A/B | 13/11 | 17/7 | <0.001 | 6.22 (2.02–19.1) | 0.001 | |
| MELD score >10, no/yes | 12/12 | 17/7 | <0.001 | 6.38 (1.95–20.8) | 0.002 | |
| ALT >40 U/L, no/yes | 9/15 | 10/15 | 0.098 | 0.43 (0.16–1.26) | 0.126 | |
| AST >40 U/L, no/yes | 9/15 | 11/7 | 0.996 | 1.00 (0.35–2.85) | 0.996 | |
| ALB <35 g/L, no/yes | 13/11 | 15/7 | 0.002 | 5.37 (1.57–18.3) | 0.007 | |
| AFP >400 ng/mL, no/yes | 6/8 | 7/15 | 0.522 | 0.73 (0.25–2.09) | 0.555 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; PVTT, portal vein tumor thrombosis; ECOG, Eastern Cooperative Oncology Group; EGV, esophageal-gastro varices; MELD, Model for End-Stage Liver Disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; AFP, alpha-fetoprotein.
Figure 1 shows the changes in WHVP, FHVP and CSPH for every patient before and after TACE. Table 3 shows, for the entire cohort, that both the median and mean values of WHVP, FHVP and HVPG decreased significantly after the TACE procedure (both P values <0.05, for comparing medians and means respectively). According to the HVPG values, patients were divided into two groups, namely, the non-CSPH group, which included 17 patients, and the remaining 7 patients with CSPH as the CSPH group. As expected, the values of WHVP, FHVP and CSPH before and after TACE for CSPH patients were significantly higher than those of the non-CSPH patients (P<0.05, Table 1). More interestingly, their absolute changes were more obvious for patients with CSPH than for the others; for example, the mean values of WHVP, FHVP and CSPH decreased by 5.5, 2.3 and 3.4 mmHg, respectively, for CSPH patients before and after TACE treatment, while the same mean values decreased by only 3.2, 1.5 and 1.7 mmHg, respectively, for non-CSPH patients. In addition, as HVPG decreased, the range of reduction in WHVP was more obvious than that of FHVP.
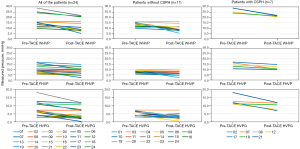
Table 3
| Variable | Total | Non-CSPH | CSPH. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-TACE | Post-TACE | P | Pre-TACE | Post-TACE | P | Pre-TACE | Post-TACE | P | |||
| WHVP, mmHg | 15.7 [11.5, 24.3] | 11.0 [8.1, 21.1] | <0.001 | 14.0 [11.0, 15.8] | 10.7 [7.9, 12.7] | 0.001 | 29.0 [24.3, 29.0] | 21.7 [21.3, 22.0] | 0.017 | ||
| 17.3±1.4 | 13.4±1.2 | <0.001 | 13.3 ±0.6 | 10.1±0.8 | 0.002 | 27.1±0.9 | 21.6±0.2 | <0.001 | |||
| FHVP, mmHg | 8.9 [7.8, 10.7] | 8.7 [4.7, 10.3] | 0.001 | 8.3 [7.5, 9.0] | 6.7 [4.7, 9.5] | 0.031 | 13.0 [10.7, 16.3] | 10.7 [10.0, 13.0] | 0.017 | ||
| 9.81±0.6 | 8.1 ±0.7 | 0.001 | 8.3±0.3 | 6.8±0.7 | 0.016 | 13.6±0.9 | 11.3±0.7 | 0.018 | |||
| HVPG, mmHg | 6.2 [3.9, 11.3] | 3.9 [2.7, 7.6] | <0.001 | 6.00 [3.03, 6.50] | 3.0 [2.7, 4.0] | 0.006 | 12.0 [11.3, 18.3] | 10.6 [7.7, 12.0] | 0.018 | ||
| 7.5±0.9 | 5.3±0.7 | <0.001 | 5.0±0.4 | 3.3±0.4 | 0.002 | 13.6±1.2 | 10.2±0.8 | 0.014 | |||
Data are in median [IQR] or mean ± S.E. DEB-TACE, drug-eluting bead TACE; CSPH, clinically significant portal hypertension; TACE, transarterial chemoembolization; WHVP, wedged hepatic vein pressure; FHVP, free hepatic vein pressure; HVPG, hepatic venous pressure gradient; IQR, interquartile range; S.E., standard error.
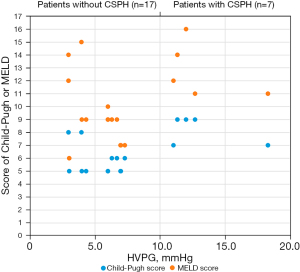
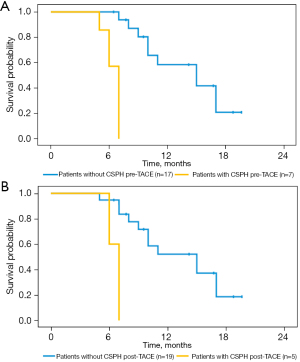
Table 1 and Figure 2 show that the HVPG varied with the patients’ liver function. The median Child-Pugh score of patients with CSPH was higher than that of patients without CSPH (9 vs. 5, P<0.001), and the median MELD scores for patients with and without CSPH were also significantly different (12 vs. 9, P=0.006). According to Kapan–Meier survival analysis, the 17 patients without CSPH had a median OS of 15.0 months, which was significantly longer than that of the 7 patients with CSPH, who had a median OS of 7.0 months (log-rank P<0.001, Figure 3A). Similarly, Cox regression analysis demonstrated that CSPH correlated with OS (HR 1.15, 95% CI: 1.02–1.29, P=0.020), and after adjusting for patients’ liver function (Child-Pugh score), CSPH could independently predict OS (adjusted HR 1.14, 95% CI: 1.01–1.29, P=0.033). After TACE therapy, HVPG was also associated with the prognoses of patients; the median OS for the 19 patients without CSPH was longer than that for the 5 other patients (15.0 vs. 7.0 months, log-rank P<0.001, Figure 3B). In summary, liver function was not only correlated with portal hemodynamics but also the most important prognostic factors of survival. Therefore, the predictive values of CSPH were adjusted by Child-Pugh score, age and ECOG in a multivariate Cox regression model, similar findings were demonstrated (adjusted HR 1.24, 95% CI: 1.04–1.48, P=0.017).
Discussion
In the present retrospective observational study, we found that hepatic hemodynamics significantly changed after DEB-TACE as treatment of unresectable HCC. Interestingly, different from the effects of cTACE, DEB-TACE seemed to decrease HVPG compared with baseline, especially for HCC patients with CSPH, for whom the changes in hepatic hemodynamics were more obvious than in those without CSPH. More importantly, the presence of CSPH significantly correlated with OS, not merely before but also after TACE. Compared with previous trials, the current study explored for the first time the correlations between the changes in HVPG and DEB-TACE therapy, and the prognostic value of HVPG before and after treatment.
The median OS of 10.0 months for all patients treated with TACE in our study was much shorter than the 19.8 months reported by a recent systematic review on TACE, which might be related to the disease conditions of the included patients (11). In our study, all patients had liver cirrhosis, and nearly 30% of them had CSPH; underlying diseases are an important prognostic factor for patients with HCC, especially those with CSPH (25,26). In addition, according to our study, the presence of CSPH increased the probability of death by 14% (adjusted HR 1.14, 95% CI: 1.01–1.29, P=0.033), and the patients with CSPH had a median OS of only 7.0 months, which was significantly shorter than the median OS of 15.0 months for the non-CSPH patients. Therefore, it could be concluded that CSPH is an important risk factor for patients with HCC, and including CSPH patients would explain the decreased survival of our study cohort.
Previous studies that investigated the changes in hepatic hemodynamics in HCC patients treated with cTACE found a transient increase in portal flow after the procedure due to the decrease in hepatic arterial blood flow, which peaked approximately 1 week after embolization and lasted for at least 2 weeks (23,24). In addition, another study reported that cTACE using lipiodol showed a marked increase in blood levels of vascular endothelial growth factor (VEGF), whereas DEB-TACE induced only a moderate VEGF response (27). However, according to a recent multicenter study comparing the imaging changes after the two types of TACE procedures for unresectable HCC, the percentage of portal vein narrowing was nearly 3-fold more common with DEB-TACE treatment than with cTACE therapy (28), which suggests that portal flow decreases after the TACE procedure, especially DEB-TACE, which was a different results from other previous studies. In the present study, WHVP, FHVP and HVPG decreased significantly after the DEB-TACE procedure, and their absolute changes were more obvious for patients with CSPH. Compared with cTACE therapy in previous studies, our differing findings might result from the embolization material used, and patient selection should also be noted. TACE is commonly recommended for patients with BCLC-B stage HCC, although patients with advanced diseases also undergo TACE in clinical practice (6). In our study, >40% of patients had PVTT, and the treatment effects of DEB-TACE on PVTT might be more obvious than those of cTACE treatment (29,30). Therefore, DEB-TACE might decrease the obstruction of the portal vein by influencing PVTT and thus relieve the pressure of the portal vein.
The present study investigated for the first time the influence of DEB-TACE therapy on hepatic hemodynamics in HCC patients and drew different conclusions from previous studies concentrating on cTACE treatment. However, there were also several limitations to our study. First, the retrospective nature and small sample size of this study might lead to some bias in the findings; prospective studies with enough participants should be designed. Second, a single-armed design with DEB-TACE therapy alone might limit the persuasiveness of our study, and comparisons with cTACE treatment should be taken into consideration in future studies. Finally, the end-point of this study needs to be detailed despite the setting of OS, and a competing risk model with tumor progression and liver failure should be used in future studies.
Conclusions
DEB-TACE treatment could influence hepatic hemodynamics in HCC patients and decrease HVPG levels, especially in patients with CSPH; in addition, the presence of CSPH was an important prognostic factor for survival. Considering that DEB-TACE could not only control tumor progression but also decrease portal hypertension and relieve underlying liver disease, future well-designed studies with large sample sizes are highly needed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-76/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-76/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-76/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-76/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by Institutional Ethics Board of The Affiliated Yantai Yuhuangding Hospital of Qingdao University (No. Yanyuyi 2018-312). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345-62. [Crossref] [PubMed]
- Global Burden of Disease Liver Cancer Collaboration. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683-91. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. [Crossref] [PubMed]
- Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36. [Crossref] [PubMed]
- Piscaglia F, Ogasawara S. Patient Selection for Transarterial Chemoembolization in Hepatocellular Carcinoma: Importance of Benefit/Risk Assessment. Liver Cancer 2018;7:104-19. [Crossref] [PubMed]
- Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol 2017;67:173-83. [Crossref] [PubMed]
- Liu A, Liu B, Duan X, et al. Development of a novel combined nomogram model integrating Rad-score, age and ECOG to predict the survival of patients with hepatocellular carcinoma treated by transcatheter arterial chemoembolization. J Gastrointest Oncol 2022;13:1889-97. [Crossref] [PubMed]
- Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64:106-16. [Crossref] [PubMed]
- Manjunatha N, Ganduri V, Rajasekaran K, et al. Transarterial Chemoembolization and Unresectable Hepatocellular Carcinoma: A Narrative Review. Cureus 2022;14:e28439. [Crossref] [PubMed]
- Angelico M. TACE vs DEB-TACE: Who wins? Dig Liver Dis 2016;48:796-7. [Crossref] [PubMed]
- Melchiorre F, Patella F, Pescatori L, et al. DEB-TACE: a standard review. Future Oncol 2018;14:2969-84. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis 2016;48:571-7. [Crossref] [PubMed]
- Yang B, Liang J, Qu Z, et al. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: A systematic review. PLoS One 2020;15:e0227475. [Crossref] [PubMed]
- Shao G, Zou Y, Lucatelli P, et al. Chinese expert consensus on technical recommendations for the standard operation of drug-eluting beads for transvascular embolization. Ann Transl Med 2021;9:714. [Crossref] [PubMed]
- Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844-55. [Crossref] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Moriyasu F, Ban N, Nishida O, et al. Portal hemodynamics in patients with hepatocellular carcinoma. Radiology 1986;161:707-11. [Crossref] [PubMed]
- Taourel P, Dauzat M, Lafortune M, et al. Hemodynamic changes after transcatheter arterial embolization of hepatocellular carcinomas. Radiology 1994;191:189-92. [Crossref] [PubMed]
- Okada K, Koda M, Murawaki Y, et al. Changes in esophageal variceal pressure after transcatheter arterial embolization for hepatocellular carcinoma. Endoscopy 2001;33:595-600. [Crossref] [PubMed]
- Elia C, Venon WD, Stradella D, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma in cirrhosis: influence on portal hypertension. Eur J Gastroenterol Hepatol 2011;23:573-7. [Crossref] [PubMed]
- Scheiner B, Ulbrich G, Mandorfer M, et al. Short- and long-term effects of transarterial chemoembolization on portal hypertension in patients with hepatocellular carcinoma. United European Gastroenterol J 2019;7:850-8. [Crossref] [PubMed]
- Kim NH, Lee T, Cho YK, et al. Impact of clinically evident portal hypertension on clinical outcome of patients with hepatocellular carcinoma treated by transarterial chemoembolization. J Gastroenterol Hepatol 2018;33:1397-406. [Crossref] [PubMed]
- Choi JW, Chung JW, Lee DH, et al. Portal hypertension is associated with poor outcome of transarterial chemoembolization in patients with hepatocellular carcinoma. Eur Radiol 2018;28:2184-93. [Crossref] [PubMed]
- Schicho A, Hellerbrand C, Krüger K, et al. Impact of Different Embolic Agents for Transarterial Chemoembolization (TACE) Procedures on Systemic Vascular Endothelial Growth Factor (VEGF) Levels. J Clin Transl Hepatol 2016;4:288-92. [Crossref] [PubMed]
- Zhang L, Sun JH, Ji JS, et al. Imaging Changes and Clinical Complications After Drug-Eluting Bead Versus Conventional Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma: Multicenter Study. AJR Am J Roentgenol 2021;217:933-43. [Crossref] [PubMed]
- Zhou TY, Chen SQ, Wang HL, et al. Safety and efficacy of drug-eluting bead transarterial chemoembolization with CalliSpheres® microsphere for hepatocellular carcinoma with portal vein tumor thrombus: a preliminary study. J Cancer 2021;12:4522-9. [Crossref] [PubMed]
- Zhao GS, Liu S, Liu Y, et al. Assessment of efficacy and prognostic factors by Gelfoam for DEB-TACE in unresectable large hepatocellular carcinoma with portal vein tumor thrombus: a multi-center retrospective study. Expert Rev Gastroenterol Hepatol 2022;16:673-80. [Crossref] [PubMed]
(English Language Editor: K. Brown)


