
Prognostic nutritional index predicts the prognosis of patients with advanced esophageal cancer treated with immune checkpoint inhibitors: a retrospective cohort study
Highlight box
Key findings
• PNI is a simple and effective biomarker for predicting the prognosis of immunotherapy in patients with advanced EC.
What is known and what is new?
• The efficacy of immunotherapy in advanced EC is variable, and there are still no effective and convenient biomarkers to identify and assess their efficacy. Biomarkers such as PD-L1 expression and TMB are hard to obtain sometimes and too expensive.
• PNI has been applied in some other cancers, but there is little study about it in EC treated with immunotherapy. Besides, it is an indicator that is very easy to detect.
What is the implication, and what should change now?
• PNI may be regarded as a useful biomarker in advanced EC patients treated with immunotherapy in the future. However, further prospective studies are warranted.
Introduction
Esophageal cancer (EC) is the seventh most common type of cancer in the world and the sixth leading cause of cancer-related death (1). China has the most cases of EC worldwide (2). At present, the prognosis of EC remains poor, mainly because most EC cases are detected at an advanced stage and the efficacies of conventional treatments are limited. In recent years, immunotherapy has provided new insight into anti-tumor treatment and has achieved gratifying results in the management of EC. As shown in a series of clinical trials including KEYNOTE181 (3), KEYNOTE590 (4), and CheckMate648 (5), immune checkpoint inhibitors (ICIs) can prolong progression-free survival (PFS) and overall survival (OS) in patients with advanced EC. Thus, immunotherapy has increasingly been applied in the first- and second-line treatment of advanced EC.
The efficacies of immunotherapy for EC vary dramatically, which poses a major challenge to screening the appropriate recipients using feasible response indicators. At present, people are still exploring effective and convenient biomarkers. Programmed cell death-Ligand 1 (PD-L1) expression is a well-known biomarker for predicting the efficacy of immunotherapy. Its positive often indicates better efficacy, but its cut-off point is not clear. Microsatellite instability-High (MSI-H) is relatively rare in patients with esophageal squamous cell carcinoma. tumor mutation burden (TMB) is expensive and difficult to meet clinical needs. From the perspective of acquisition, blood biomarkers are more popular than tumor tissue biomarkers. Therefore, if they can have excellent prognostic value, they will have a broader prospect. Malnutrition can adversely affect the outcomes of cancer patients by reducing treatment tolerability, increasing side effects, and thus decreasing efficacy (6). The prognostic nutritional index (PNI), a parameter combining serum albumin (ALB) concentration and absolute lymphocyte count, reflects the immune and nutritional status of tumor patients and has been shown to be a positive prognostic marker for various malignant tumors (7-11). However, it is mainly based on retrospective research and its clinical significance and prognostic value for advanced EC after immunotherapy remain uncertain. Here we retrospectively investigated the effect of PNI on OS and PFS in advanced EC patients treated with immunotherapy. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-48/rc).
Methods
Study design
Our study is a retrospective cohort study from September 2018 to May 2022. The number of cases meeting the inclusion criteria during the study period determined the sample size. The primary objective was to correlate PNI with PFS, defined as the time from the initiation of treatment with ICIs to disease progression detected by imaging (contrast-enhanced CT) or laboratory indicators (e.g., CEA and CA199). OS was defined as time from ICI treatment to death from any cause. The primary endpoint was PFS; the secondary endpoint was OS. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of the 900th Hospital of the Joint Logistics Team (No. 2022-031) and individual consent for this retrospective analysis was waived.
Patient information
A total of 83 patients with advanced EC who received immunotherapy in the 900th Hospital of the Joint Logistics Team from September 2018 to May 2022 were enrolled in this retrospective analysis. The inclusion criteria were as follows: (I) aged ≥18 years; (II) with an Eastern Cooperative Oncology Group (ECOG) performance status score of ≤1; (III) with a histopathologically confirmed diagnosis of esophageal malignancy; (IV) with locally advanced or metastatic/recurrent inoperable cancer; (V) ≤1 assessable lesion before treatment [according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1]; and (VI) without any co-infection or serious systemic disease before treatment. The exclusion criteria were as follows: (I) complicated by other malignancies in addition to cured skin basal cells, squamous cell carcinoma, or any other carcinoma in situ; (II) developing any abnormal myeloproliferative and other hematopoietic disorders before treatment; (III) with human immunodeficiency virus (HIV) infection or viral hepatitis that requires treatment; and (IV) with other serious systemic diseases require medical intervention. Accordingly, 5 patients were excluded: 3 due to incomplete baseline data and 2 due to the presence of other malignancies. Thus, 78 patients entered the final analysis (Figure 1).
Treatments
Participants received intravenous anti-programmed cell death protein 1 (PD-1) drugs every 3 weeks, with or without chemotherapy and/or radiotherapy, until disease progression, unacceptable toxicity, or patient refusal. The ICIs used included camrelizumab, pembrolizumab, nivolumab, sintilimab, tislelizumab, and toripalimab. The therapeutic dose was 240 mg every 3 weeks for tislelizumab and 200 mg every 3 weeks for the remaining ICIs. Tumor responses were assessed every 6–8 weeks using contrast-enhanced computed tomography (CT) according to the RECIST (12).
The 3-week adjuvant or palliative chemotherapy regimens comprised the following: (I) fluorouracil plus cisplatin: cisplatin 60–80 mg/m2, IV, d1; fluorouracil 1,000 mg/m2 per day, IV, d1–d5. (II) paclitaxel plus cisplatin: paclitaxel 150–175 mg/m2, IV, d1 or 80 mg/m2, IV, d1 and d8; cisplatin 60–75 mg/m2, IV, d1 or d2. (III) docetaxel plus platinum: docetaxel 60–75 mg/m2, IV, d1 or 30–35 mg/m2, IV, d1–d2; cisplatin 70 mg/m2, IV, d1 or nedaplatin 50 mg/m2, IV, d1. and (IV) docetaxel monotherapy: docetaxel 60–75 mg/m2, IV, d1, repeated every 3 weeks. Considering the toxicities of combination therapies, we did not use any combination containing 3 or more chemotherapeutic drugs. Participants received 2 cycles of concurrent chemotherapy regimens, which included cisplatin (25 mg/m2 IV, d1–d3, every 3 weeks) plus paclitaxel (135 mg/m2 IV, d1, every 3 weeks).
Radiotherapy regimens included radical concurrent chemoradiotherapy at doses of 50–60 Gy and postoperative adjuvant radiotherapy at doses of 45–50.4 Gy (R0 resection was achieved in all cases), with radiation therapy divided into 28 fractions (total dose: 50.4 Gy).
Data collection
Data were collected through electronic medical records and telephone follow-up. The baseline characteristics included demographic data (age and sex), height, weight, degree of tumor differentiation, disease status (presence or absence of distant metastases), site of metastasis, treatment line, type of ICIs used, and laboratory findings. The laboratory test results within 10 days prior to the start of ICI treatment were recorded, including absolute lymphocyte count and ALB level. The following formulae were applied: body mass index (BMI) = body weight (kg)/height2 (m2); prognostic nutritional index (PNI) = ALB (g/L) + 5 × total lymphocyte count (109/L) (13).
Statistical analysis
The critical values for PNI and BMI were calculated using the X-Tile software (Brady Memorial Laboratory, New Haven, CT, USA). Survivals were analyzed using the Kaplan–Meier method, and the comparisons of these survival curves were based on the log-rank test. Cox proportional hazards regression model was used to analyze the factors affecting PFS and OS and to calculate the hazard ratio (HR) and the 95% confidence interval (CI). The multivariate analysis included statistically significant factors (P<0.1) that had been identified in the univariate analysis and was performed by using the forward elimination model. All P values reported are 2-tailed, and values of P<0.05 were considered statistically significant. The statistical analyses were performed using the software SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R language (version 4.2.1; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline pathological features
Retrospective analysis of these 78 patients showed the median age was 58 years, the vast majority (83.3%) of these patients were males, and esophageal squamous cell carcinoma accounted for 93.6% (Table 1). Distant metastases were common (69.2%), among which extramediastinal lymph nodes (50%) and lungs (30.8%) were the most commonly affected sites. About half (51.3%) of the patients received first-line treatment. Camrelizumab was the most commonly used anti-PD-1 drug.
Table 1
| Variables | Number (%) or median [range] |
|---|---|
| Age, years | 58 [46–87] |
| <60 | 42 (53.8) |
| ≥60 | 36 (46.2) |
| Sex | |
| Male | 65 (83.3) |
| Female | 13 (16.7) |
| Pathological type | |
| Squamous carcinoma | 73 (93.6) |
| Others | 5 (6.4) |
| Metastasis | |
| No | 24 (30.8) |
| Yes | 54 (69.2) |
| Site of metastasis | |
| Lung | 24 (30.8) |
| Liver | 16 (20.5) |
| Bone | 7 (9.0) |
| Lymph node outside chest | 39 (50.0) |
| Others | 4 (5.1) |
| PD-L1 status | |
| CPS <5 | 8 (10.3) |
| CPS ≥5 | 13 (16.7) |
| Unknown | 57 (73.1) |
| Histological grade | |
| Poorly differentiated | 35 (44.9) |
| Moderately differentiated | 27 (34.6) |
| Well differentiated | 16 (20.5) |
| Treatment line | |
| 1st line | 40 (51.3) |
| ≥2nd line | 38 (48.7) |
| Immune checkpoint inhibitors | |
| Camrelizumab | 41 (52.6) |
| Nivolumab | 1 (1.3) |
| Pembrolizumab | 4 (5.1) |
| Sintilimab | 19 (24.4) |
| Tislelizumab | 8 (10.3) |
| Toripalimab | 5 (6.4) |
PD-L1, programmed death ligand-1; CPS, combined positive score.
Impact of PNI on prognosis
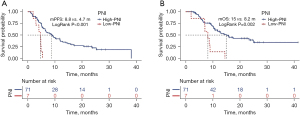
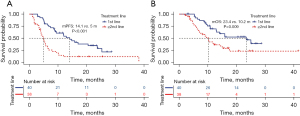
The OS and PFS were 13 months and 7.4 months, respectively, in these 78 patients (Figure 2). The cut-off values for BMI and PNI were set at 18.8 and 40.6, respectively, by using the X-Tile software. As shown in Figure 3, the median PFS and OS in the high-PNI group were significantly longer than those in the low-PNI group (8.8 vs. 4.7 months, P<0.001; 15 vs. 8.2 months, P=0.002). In addition, PFS and OS were significantly prolonged after first-line therapy (Figure 4).

Influencing factors of PFS and OS
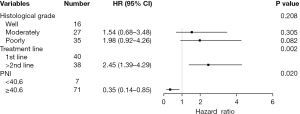
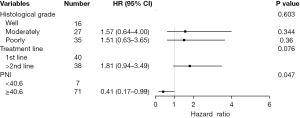
We used a Cox proportional hazards regression model to analyze the potential influencing factors of PFS and OS. Univariate analysis showed that treatment line (HR =2.77, 95% CI: 1.63–4.73, P<0.001; HR =2.21, 95% CI: 1.20–4.07, P=0.011) and PNI value (HR =0.23, 95% CI: 0.10–0.55, P=0.001; HR =0.28, 95% CI: 0.12–0.65, P=0.003) were significantly correlated with PFS and OS. Meanwhile, poor tumor differentiation (HR =2.22, 95% CI: 1.04–4.72, P=0.040) was significantly associated with poor PFS; however, age, gender, presence or absence of distant metastases, baseline BMI, and programmed death ligand-1 (PD-L1) status were not significantly correlated with PFS or OS (all P>0.05) (Table 2).
Table 2
| Variables | PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Sex (female) | 0.70 (0.32–1.54) | 0.373 | 0.67 (0.26–1.70) | 0.395 | |||||||
| Age (≥60 years) | 0.84 (0.50–1.42) | 0.521 | 0.70 (0.38–1.29) | 0.248 | |||||||
| Metastasis (no) | 1.15 (0.67–1.99) | 0.607 | 0.88 (0.48–1.65) | 0.699 | |||||||
| Histological grade | |||||||||||
| Moderately | 2.11 (0.95–4.67) | 0.065 | 1.54 (0.68–3.48) | 0.352 | 2.23 (0.92–5.41) | 0.076 | 1.57 (0.64–4.00) | 0.344 | |||
| Poorly | 2.22 (1.04–4.72) | 0.040 | 1.98 (0.92–4.26) | 0.082 | 1.70 (0.71–4.05) | 0.235 | 1.51 (0.63–3.65) | 0.360 | |||
| PD-L1 expression (CPS ≥5) | 1.29 (0.46–3.63) | 0.636 | 1.83 (0.50–6.67) | 0.361 | |||||||
| Treatment line (≥2nd line) | 2.77 (1.63–4.73) | <0.001 | 2.45 (1.39–4.29) | 0.002 | 2.21 (1.20–4.07) | 0.011 | 1.81 (0.94–3.49) | 0.076 | |||
| BMI (≥18.8 kg/m2) | 0.72 (0.39–1.32) | 0.293 | 0.62 (0.32–1.20) | 0.157 | |||||||
| PNI (≥40.6) | 0.23 (0.10–0.55) | 0.001 | 0.35 (0.14–0.85) | 0.020 | 0.28 (0.12–0.65) | 0.003 | 0.41 (0.17–0.99) | 0.047 | |||
PD-L1, programmed death ligand-1; CPS, combined positive score; PFS, progression-free survival; OS, overall survival; CI, confidence interval; HR, hazard ratio; BMI, body mass index; PNI, prognostic nutrition index.
Multivariate analysis of the above statistically significant indicators showed that the treatment line remained an independent predictor of PFS (HR =2.45, 95% CI: 1.39–4.29, P=0.002), whereas baseline high PNI was associated with longer PFS (HR =0.35, 95% CI: 0.14–0.85, P=0.02) and OS (HR =0.41, 95% CI: 0.17–0.99, P=0.047) (Table 2, Figures 5,6).
Discussion
Although ICIs have become a new treatment for EC, the factors affecting their efficacy are still unclear. Although PD-L1 expression is currently a well-known biomarker for assessing the efficacy of immunotherapy, a meta-analysis involving immunotherapy for multiple cancers revealed that immunotherapy can significantly improve patient outcomes regardless of PD-L1 status; however, a study has shown that immunotherapy is less effective in patients with negative PD-L1 expression (14). Thus, a variety of factors can affect the efficacy of ICIs. Many biomarkers for immunotherapy have been available, including microsatellite instability/mismatch repair deficiency (MSI/dMMR), tumor mutation burden (TMB), tumor infiltrating lymphocytes (TILS), tertiary lymphoid structures (TLS), PNI, neutrophil-lymphocyte ratio (NLR), and lactate dehydrogenase (LDH) (15). However, immature detection technology, difficulty in obtaining specimens, and high prices limit the application of these biomarkers in clinical practice.
Thus, the roles of nutrition- and inflammation-related indicators in immunotherapy have increasingly been recognized. BMI is a simple and easily available nutrition indicator. In a retrospective analysis of metastatic melanoma, McQuade et al. (16) showed that high BMI was positively correlated with improved prognosis. Similarly, Martini et al. (17) found in a phase 1 trial that higher BMI was associated with longer PFS (HR =0.96, 95% CI: 0.92–1.00; P=0.03) and OS (HR =0.92, 95% CI: 0.87–0.97; P=0.001) in patients receiving immunotherapy for advanced solid tumors. However, in another study of metastatic melanoma treated with ICIs, BMI was not significantly associated with OS or PFS (18). There is still no robust evidence base that unravels the relationship between BMI and immunotherapy efficacy. In our current study, we also did not find an association between BMI and prognosis, which needs to be further explored by prospective studies with larger sample sizes.
PNI includes ALB and lymphocyte count, which reflect the nutritional and immunological status, respectively. Up to 90% of upper gastrointestinal cancer patients have nutritional problems (19), which is mainly caused by reduced food intake (due to dysphagia, decreased appetite, and other factors) and increased nutrition consumption by tumors. Malnutrition has long been considered a risk factor for tumor prognosis (20). The tumor microenvironment is another important factor in tumorigenesis. The inflammatory factors can substantially affect tumor cell growth, angiogenesis, and tumor invasion/metastasis by recruiting T lymphocytes, tumor-associated macrophages, and circulating cytokines (21). Lymphocytes play a key role in adaptive immunity. A decrease in the number of lymphocytes may reduce the ability of the immune system to inhibit tumor cell proliferation and metastasis, thereby promoting tumor progression (22,23). The combination of these 2 factors can better reflect the host conditions. Onodera et al. (13) initially used PNI to assess immunotrophic status and surgical risks in patients undergoing gastrointestinal surgery. Subsequently, PNI has been applied in gastric cancer (8), lung cancer (9), liver cancer (10), pancreatic cancer (11), and many other cancers, and research on EC has mostly related to surgical prognosis (24,25). In a meta-analysis of 11 studies involving 3,425 patients with EC (26), low PNI value was associated with poor prognosis and was an independent predictor of PFS and OS; in addition, male gender, older age, and advanced tumors were associated with low PNI, but PNI was not significantly associated with tumor grade or distant metastasis. A retrospective case-control study with an age- and gender-matched control cohort indicated that high Onodera’s PNI (>33) was the most significant nutritional predictors of better survival (27). In our current study, we retrospectively analyzed the potential influencing factors affecting immunotherapy in patients with advanced EC, and the results were consistent with the above studies: the low baseline PNI value before treatment was associated with poor prognosis and was an independent predictor of PFS and OS. Since lymphocyte count and ALB level are routinely tested, PNI is a much more convenient and economical indicator compared to predictive markers that require the harvesting of histological specimens. For patients with low baseline PNI, timely and individualized nutritional and immunological interventions may improve the prognosis of patients.
Furthermore, PFS was significantly longer after first-line treatment than after second-or-later-line treatment (median PFS: 14.1 vs. 5 months), which was further confirmed by multivariate analysis. The KEYNOTE590 study (4) laid the foundation for the first-line immunotherapy for advanced EC, and the CheckMate648 study (5) found that dual immunotherapy could significantly prolong survivals in patients with unresectable advanced or metastatic esophageal squamous cell carcinoma. Nevertheless, more studies should be carried out to optimize the first-line treatment of advanced EC. In addition, univariate analysis of the degree of tumor differentiation showed that poor differentiation was significantly associated with short PFS, which, however, was not found in the multivariate analysis. In a study of immunotherapy for advanced head and neck squamous cell carcinoma, the response rate to immunotherapy was 5.35-fold higher in the high-grade group (moderately- and well-differentiated) than that in the lower-grade group (poorly-differentiated), suggesting tumor histological grade was an independent predictor of immunotherapy response (28). Poor tumor differentiation implies a high degree of malignancy and may be associated with a poor prognosis, which also needs to be further investigated in clinical settings.
Our research had some limitations. First, due to its single-center, retrospective design, our current study had a small sample size and might have involved selection/information biases. Second, since most of our participants were not tested for PD-L1 status, we did not compare its prognostic value with PNI, and its impact on prognosis might also be affected by the small sample size. Therefore, further prospective studies with large sample sizes are warranted.
Conclusions
Low PNI is associated with poor PFS and OS and can be used as a predictor of immunotherapy response in EC patients. For patients with low PNI values, their nutritional and immunological status should be carefully monitored and actively intervened, in order to improve survival outcomes.
Acknowledgments
Funding: This work was supported by Three-Year Plan of Shanghai Municipal Health Commission (GWV-10.2-YQ27, 20214Y0012) and National Military Grants (AEP17J001).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-48/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-48/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of the 900th Hospital of the Joint Logistics Team (No. 2022-031) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Morgan E, Soerjomataram I, Rumgay H, et al. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology 2022;163:649-658.e2. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. Erratum in: Lancet 2021;398:1874. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esoph-ageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemo-therapy in cancer patients. Eastern Cooperative Oncology Group. The American journal of medicine 1980;69:491-7. [Crossref] [PubMed]
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prog-nostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Can-cer 2011;47:2633-41. [Crossref] [PubMed]
- Namikawa T, Yokota K, Tanioka N, et al. Systemic inflammatory response and nutri-tional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today 2020;50:1486-95. [Crossref] [PubMed]
- Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and im-mune-related adverse events in advanced non-small cell lung cancer treated with PD-1 in-hibitors. Cancer Immunol Immunother 2020;69:1813-22. [Crossref] [PubMed]
- Wang D, Hu X, Xiao L, et al. Prognostic Nutritional Index and Systemic Im-mune-Inflammation Index Predict the Prognosis of Patients with HCC. J Gastrointest Surg 2021;25:421-7. [Crossref] [PubMed]
- Fu M, Yu L, Yang L, et al. Predictive value of the preoperative prognostic nutritional index for postoperative progression in patients with pancreatic neuroendocrine neoplasms. Front Nutr 2022;9:945833. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal sur-gery of malnourished cancer patients. Nihon Geka Gakkai zasshi 1984;85:1001-5.
- Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 2018;362:k3529. [Crossref] [PubMed]
- Grecea M, Soritau O, Dulf D, et al. Potential Biomarkers for the Efficacy of PD-1-PD-L Blockade in Cancer. Onco Targets Ther 2021;14:5275-91. [Crossref] [PubMed]
- McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and out-comes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018;19:310-22. [Crossref] [PubMed]
- Martini DJ, Kline MR, Liu Y, et al. Adiposity may predict survival in patients with ad-vanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer 2020;126:575-82. [Crossref] [PubMed]
- Rutkowski P, Indini A, De Luca M, et al. Body mass index (BMI) and outcome of metastatic melanoma patients receiving targeted therapy and immunotherapy: a multicenter international retrospective study. J Immunother Cancer 2020;8:e001117. [Crossref] [PubMed]
- Furness K, Silvers MA, Savva J, et al. Long-term follow-up of the potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: a pilot randomised trial. Support Care Cancer 2017;25:3587-93. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, inva-sion, and metastasis. Cell 2006;124:263-6. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- Kitayama J, Yasuda K, Kawai K, et al. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol 2010;5:47. [Crossref] [PubMed]
- Nakatani M, Migita K, Matsumoto S, et al. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Hirahara N, Tajima Y, Fujii Y, et al. Preoperative Prognostic Nutritional Index Predicts Long-Term Surgical Outcomes in Patients with Esophageal Squamous Cell Carcinoma. World J Surg 2018;42:2199-208. [Crossref] [PubMed]
- Xue Y, Zhou X, Xue L, et al. The role of pretreatment prognostic nutritional index in esophageal cancer: A meta-analysis. J Cell Physiol 2019;234:19655-62. [Crossref] [PubMed]
- Morelli C, Formica V, Patrikidou A, et al. Nutritional index for immune-checkpoint inhibitor in patients with metastatic gastro-esophageal junction/gastric cancer. J Gastrointest Oncol 2022;13:2072-81. [Crossref] [PubMed]
- Alkhatib HH, Maroun CA, Amin N, et al. Tumor Histological Grade and Immunotherapy Response in Patients With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol Head Neck Surg 2022;148:540-6. [Crossref] [PubMed]
(English Language Editor: J. Jones)



