
Outcome analysis in patients with metastatic gastroenteropancreatic neuroendocrine tumors receiving peptide receptor radionuclide therapy with Lu-177-DOTATATE
Highlight box
Key findings
• PRRT with Lu-177-DOTATATE in the treatment of metastatic GEP-NET seems to be well tolerated without increasing patient symptomatic burden. SIRT prior to PRRT and the lack of SSA after PRRT seems to be detrimental for survival.
What is known and what is new?
• Improved response rates and extended progression-free survival with PRRT and LAR compared to LAR alone have been demonstrated in the NETTER-1 trial. HRQoL in the DOTATATE arm was significantly improved compared to placebo.
• The present study reports real-life data within an ENETS center and analyzed the role of PRRT in non-pancreatic and pancreatic NET. It adds to the favorable results of previous trials, confirming low toxicity and sheds light on potentially beneficial treatment sequences.
What is the implication, and what should change now?
• The NETTER-2 and COMPETE trials are two prospective ongoing trials evaluating whether PRRT may be employed as first-line treatment.
Introduction
Neuroendocrine tumors (NET) comprise a heterogenous group of rare tumors originating from cells of the neural crest or the diffuse neuroendocrine system. These cells resemble both neurons and endocrine hormone-producing cells and may release hormones such as somatostatin, serotonin, and neuropeptides (1,2).
NET are distinguished from neuroendocrine carcinomas (NEC) based on their differentiation and molecular differences (3).
Two thirds of NET originate in the gastroenteropancreatic (GEP) system and contribute to approximately 1% to 2% of all tumors of the gastroenterohepatic system (4). Approximately 20% of NET are metastatic when they are first diagnosed (5).
The morphological and histological classification of GEP-NET determines the therapeutic approach and the prognosis (6). Somatostatin receptors (SSTR) are overexpressed in NET, particularly SSTR2, and hence SSTR2-expressing tumors may be indicators of NET. Up to 90% of GEP-NETs express SSTR2 targeted by somatostatin analogs (SSA) (7-9). If the tumor is deemed resectable even in an advanced setting, surgery will be chosen frequently as even in metastatic disease, surgical resection can help to cytoreduce functional tumors with subsequent improvement of symptoms although limitations of surgical approaches are evident. According to the recommendations of the European Society for Medical Oncology (ESMO) guidelines, even advanced intestinal NET are resected to prevent complications such as intestinal obstruction or ischemia (10). Watchful waiting is the strategy for low grade-non-functional tumors, while advanced progressive tumors are initially treated with SSA. If the tumor progresses further after SSA treatment, the consecutive therapy depends on the location of the tumor; in this context, pancreatic and non-pancreatic tumors have distinct therapeutic approaches. Treatment of non-pancreatic NET primarily involves therapies directed at the liver, while pancreatic NET are systemically treated with chemotherapy or mechanistic target of rapamycin (mTOR) inhibitors (6).
Peptide receptor radionuclide therapy (PRRT) is a form of radiotherapy in which a radiation activity is distributed to tumor cells by radioactively labelled peptides (11). It may be selected for patients with metastatic tumors, to cytoreduce them, to improve symptoms, avoid future complications and possibly to enhance survival rates. The peptide, a somatostatin analogue, functions as the vector that facilitates the delivery of the radionuclide to the cell by binding to SSTR2. Lu-177-DOTATATE is a radio conjugate consisting of radio labelled Lutetium (Lu-177), the chelating agent tetra-azacyclododecane tetraacetic acid (DOTA), and the tyrosine containing somatostatin analogue Tyr3-octreotate (TATE) (12). Lu-177 is a medium energy β-emitter, maximum = 0.5 MeV, with a half-life (T1/2) of almost seven days (13) [Table 1 (14)], this radionuclide is suitable for treatment of small tumors owing to the comparatively low-range emission of Lu-177 with maximum tissue penetration of 2 mm (15). DOTA serves as a chelator to combine the radionuclide with the somatostatin analogue.
Table 1
| Decay calculation (t1 → t2) for Lu-177 |
| t1 = 6.647 d → t2 = 13.294 d |
| Nuclide → Lu-177 |
| (T½) → (6.647 d) |
| t (d) ↓ → A(t) ↓ |
| 0 → 1E3 |
| 6.647E0 → 5E2 |
| 1.3294E1 → 2.5E2 |
| t or Δt (d) = time or elapsed time in days (d) since beginning date |
| T½ = half-life |
| A(t) = nuclide activity (parent or daughter) at time (t) |
| E0 − E2 = excited levels |
The TATE component of Lu-177-DOTATATE binds to somatostatin receptors (SSTR) on the cell surface, with particularly high affinity for SSTR-2 (16). The binding affinity of the TATE peptide to the somatostatin receptors is superior to other peptides tested for PRRT, such as Tyr3-octreotide and 1-Nal3-octreotide (13). This is particularly relevant for the use of PRRT as a therapy for pancreatic GEP-NET, with a high expression of the SSTR-2 receptor in this tissue (17).
PRRT has been employed in oncological practice for more than 20 years (18-22). Lu-177-DOTATATE is typically administered as a sequence of 4 cycles. This therapy is associated with several side effects, including nephrotoxicity and hematotoxicity, which warrants confirmation of sufficient kidney function and bone marrow stores of the patients before the initiation of the therapy (23). Up to 80% of patients with both well-differentiated and metastatic NET appear to respond to PRRT (23). The prognosis hereby correlates directly with the initial response to the therapy. The clinical efficacy of PRRT in the treatment of NET has been demonstrated for different NET entities, with a favorable prognosis observed for pancreatic NET and NET originating in the duodenum, while the prognosis for NET of unspecified origin is worse (24).
In the NETTER-1 trial, patients with well-differentiated (G1/G2) GEP-NET of the midgut were treated with PRRT with Lu-177-DOTATATE and compared to patients treated with the SSA octreotide long-acting repeatable (LAR). PRRT was associated with longer progression-free survival, improved quality of life and higher response rate compared to LAR treated patients. In addition, PRRT was successfully employed after SSA failure for SSTR2-positive G1 and G2 tumors (25).
The aim of the present study was to assess the outcome of metastatic GEP-NET patients after treatment with Lu-177-DOTATATE, specifically the potential impact of sequencing PRRT and comparison of the effects on post-PRRT treatments on mortality and health-related quality of life (HRQoL). We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-874/rc).
Methods
Study design
The study consisted of a retrospective analysis by extracting data from the medical records of patients with GEP-NETs who had been treated with Lu-177-DOTATATE at a clinic for nuclear medicine between 2012 and 2017 using the electronical hospital register KISIM. All patients treated in this timespan at our facility have been screened for eligibility in this study. At this time period, the clinic was the only available certified ENETS Center of Excellence in Switzerland.
Patients
Patients with GEP-NETs who had been treated with at least three cycles of Lu-177-DOTATATE were included in the study.
Inclusion and exclusion criteria
Patients were either previously treated at the clinic or referred to the tertiary center for PRRT with or without prior treatment. Patients with non-gastrointestinal NET were excluded from the study cohort (in total 9 patients with meningioma and 6 patients with pulmonary NET). Patients with poorly differentiated G3 tumors were excluded. All other patients with GEP-NET were eligible for analysis and data were extracted from electronic patient files.
PRRT with Lu-177-DOTATATE
Radiation safety management concerning the use of Lu-177-DOTATATE was rigorously observed and all treatment cycles performed by trained personnel at the clinic. According to the manufacturer’s treatment protocols, the injectable somatostatin analogue is administered at an activity of 4× 7,400 MBq at an interval of 8 weeks, although in a very limited number of cases the delivered activity has been adapted to patient’s overall health status and renal function. Prophylactic amino acids (1,000 mL lysine/arginine 0.25%) and antiemetics (16 mg Mephameson i.v. and 0.25 mg Aloxi i.v.) were administered beforehand.
Most patients were discharged 48 h after the injection and once the reference activity was less than 5 µSv/h at 1 meter distance.
Questionnaire
Patient symptomatic burden (fatigue, insomnia, loss of appetite, abdominal pain, nausea, emesis, diarrhea, weight loss) were assessed before and during follow-up consultations 4 weeks after treatment cycles with our own center-based questionnaire form. Data on these parameters was extracted retrospectively from patient records and were assessed before and after PRRT treatment as presence of a parameter at the time of answering the questionnaire.
Outcome parameters
To determine factors influencing the success of PRRT therapy and survival, data on pre- and post-PRRT treatments [selective internal radiation therapy (SIRT), somatostatin analogue therapy (SSA), tyrosine kinase inhibitors, everolimus, or chemotherapy] was obtained and the time-point of PRRT in the therapeutic sequence was analyzed. Eight weeks after PRRT, data on blood parameters, patient symptomatic burden were collected. Disease specific survival as well overall survival data were evaluated at the end of the follow-up period.
Ethical statement
This study was approved and accepted by the Swiss Ethics Committee (No. BASEC-Nr. 2016-01575). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Included patients approved and signed the written General Consent form so that their specific non-coded data could be extracted from the hospital’s electronic patient register. The data were then recorded as coded and anonymous data in chart review and used for statistical analysis.
Statistical analysis
Statistical analyses were performed using the SAS software (SAS Institute, Cary, NC). Clinical characteristics of patients were compared. Pearson’s chi-square test was used for categorical data and Mann-Whitney U test was for non-parametric comparisons. Univariable and multivariable binary regression analysis were used to evaluate the association between different potential factors. Statistical significance was set at P<0.05.
Results
Patient characteristics
A total of 41 patients (23 women and 18 men) were included in this retrospective analysis. The youngest patient was 13 years old at the beginning of PRRT, the oldest 83 years old. The average age of the patients was 58.4 years (Table 2).
Table 2
| Characteristics | Values |
|---|---|
| Male, n [%] | 18 [43.9] |
| Female, n [%] | 23 [56.1] |
| Age at first diagnosis (years), mean (range) | 54.7 (13 to 83) |
| Age at start of PRRT (years), mean (range) | 58.4 (15 to 84) |
| ECOG-Performance Score, n [%] | |
| 0 | 36 [90] |
| 1 | 1 [2.5] |
| 2 | 1 [2.5] |
| 3 | 1 [2.5] |
| 4 | 1 [2.5] |
| NET type, n [%] | |
| P-NET | 18 [43.9] |
| GE-NET | 23 [56.1] |
| NET grade, n [%] | |
| G1 | P-NET: 0; GE-NET: 8 [36] |
| G2 | P-NET: 14 [74]; GE-NET: 10 [45] |
| G3 | P-NET: 4 [21]; GE-NET: 1 [5] |
| Unknown | P-NET: 1 [5]; GE-NET: 3 [14] |
PRRT, peptide receptor radionuclide therapy; ECOG, Eastern Cooperative Oncology Group; NET, neuroendocrine tumors; P-NET, pancreatic neuroendocrine tumors; GE-NET, gastric and enteric NET including large intestine, rectum, paraganglioma.
Change in body weight after PRRT compared to baseline was plotted for each patient. 9 patients lost between 2 and 13 kg (mean weight loss 5.1 kg). Ten patients gained weight (mean weight gain 4.4 kg).
Eastern Cooperative Oncology Group (ECOG) scores of the patients were compared before (t0) and after (tx) PRRT and by primary tumor site (pancreas versus other tumor types). The distribution of ECOG scores did not significantly differ between different tumor entities (Table 3).
Table 3
| ECOG | 0 | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|---|
| ECOG t0 | ||||||
| P-NET | 16 | 0 | 0 | 1 | 0 | 17* |
| GE-NET | 20 | 1 | 1 | 0 | 1 | 23 |
| Total | 36 | 1 | 1 | 1 | 1 | 40 |
| ECOG tx | ||||||
| P-NET | 16 | 0 | 0 | 1 | 0 | 17 |
| GE-NET | 19 | 2 | 1 | 0 | 1 | 23 |
| Total | 35 | 2 | 1 | 1 | 1 | 40 |
*, data for one P-NET patient missing. ECOG, Eastern Cooperative Oncology Group; PRRT, peptide receptor radionuclide therapy; NET, neuroendocrine tumors; P-NET, pancreatic neuroendocrine tumors; GE-NET, gastric and enteric NET including large intestine, rectum, paraganglioma.
Patient symptomatic burden
Figure 1 shows the frequency of parameters that were absent before and after PRRT (0/0), those that were absent before treatment but present after PRRT (0/1), those that were present both at baseline and after PRRT (1/1), and those that were present before PRRT but not after the treatment (1/0).
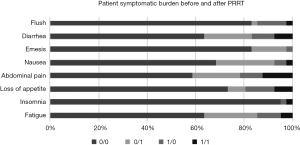
Twenty-six patients did not experience fatigue either before or after PRRT, 9 patients reported this symptom after PRRT but not before, for 4 patients this symptom was no longer present after PRRT, and for 2 patients the symptom was present at both baseline and after PRRT. Insomnia was experienced by only 2 of the 41 patients, of which one experienced the symptom before but not after PRRT, the other at both time points. Thirty patients did not experience a loss of appetite at either of the two time points, while 3 patients reported this symptom only after PRRT, 5 patients only before PRRT, and for 3 patients this symptom was present both before and after PRRT. Abdominal pain was experienced by 17 of the 41 patients, with 8 patients reporting it only after PRRT, 4 patients only before PRRT, and for 5 patients this symptom was present at both time points. 28 patients stated that nausea was no issue either before or after PRRT, while 10 patients experienced this symptom only after PRRT. For 2 patients, nausea disappeared after PRRT, and for 1 patient the symptom was present and did not change after PRRT. Seven of the 41 patients experienced emesis, with 6 patients reporting the symptom after PRRT only and one patient only before PRRT. Diarrhea was an issue for 15 of the 41 patients, while 26 patients did not experience this symptom either at baseline or after PRRT. In 4 patients, diarrhea was ameliorated by PRRT, in 8 patients it occurred only after PRRT, and in 3 patients, diarrhea was present both before and after PRRT. Seven of the 41 patients experienced flush either before PRRT only (5 patients), after PRRT only (1 patient), or both before and after PRRT (1 patient). Generally, symptoms were mild and no general effect of PRRT could be deduced.
Blood parameters
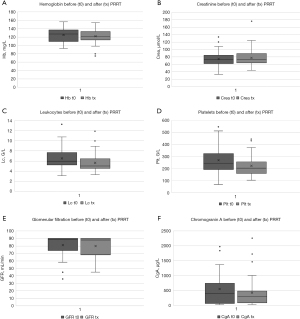
Blood parameters (hemoglobin, creatinine, glomerular filtration, chromogranin A, platelet counts, leukocyte counts) were assessed prior to any PRRT cycle and after completion of all PRRT cycles of an individual patient and the values plotted for each patient (Figure 2). Average hemoglobin values were 125.4 mg/L before PRRT and 122.3 mg/L after PRRT (Figure 2A). The difference between before and after measurements was not significant (P=0.201). Creatinine amounted to an average of 73.8 µmol/L before PRRT and 77.7 µmol/L after PRRT, with no significant differences observed after PRRT compared to baseline (P=0.146, Figure 2B). Both average leukocyte counts (6.6 G/L before PRRT, 5.6 G/L after PRRT, P<0.01, Figure 2C) and platelet counts (269.9 G/L before PRRT, 216.7 G/L after PRRT, P<0.001, Figure 2D) were significantly decreased after PRRT. Glomerular filtration rate or chromogranin A levels did not significantly differ before and after PRRT measurements (P=0.519 and P=0.475 respectively, Figure 2E,2F).
Blood parameters were also compared in a subgroup analysis between patients with pancreatic tumors and gastric and enteric NET including large intestine, rectum, paraganglioma (GE-NET) and differences between both groups were assessed with a Mann-Whitney U test (Figure 3). Biochemical values were collected prior to initiation of PRRT and after completion of all PRRT cycles. There were no significant differences in blood parameters depending on the tumor entity (Hb t0: P=0.125, IRQ: 111–136.5; Hb tx: P=0.162, IQR: 114.25–132.5; Lc t0: P=0.712, IQR: 5.3–8.0; Lc tx: P=0.607, IQR: 4.5–6.43; Plt t0: P=0.556, IQR: 189.5–339; Plt tx: P=0.158, IQR: 154.8–251.5; GFR t0: P=0.347, IQR: 75–90; GFR tx: P=0.183, IQR: 68.8–90; Crea t0: P=0.774, IQR: 62.3–80; Crea tx: P=0.530, IQR: 63.2–87.3) (Table 4).
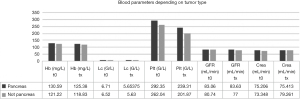
Table 4
| Parameter | P value | IQR |
|---|---|---|
| Hb at t0 | 0.125 | 111–136.5 |
| Hb at tx | 0.162 | 114.25–123.5 |
| Lc at t0 | 0.712 | 5.3–8.0 |
| Lc at tx | 0.607 | 4.5–6.43 |
| Plt at t0 | 0.556 | 189.5–339 |
| Plt at tx | 0.158 | 154.8–251.5 |
| GFR at t0 | 0.347 | 75–90 |
| GFR at tx | 0.183 | 68.8–90 |
| Crea at t0 | 0.774 | 62.3–80 |
| Crea at tx | 0.530 | 63.2–87.3 |
IQR, interquartile range; Hb, haemoglobin; Lc, leukocytes; Plt, platelets; GFR, glomerular filtration rate; Crea, creatine; t0, prior treatment; tx, after treatment.
Time point of PRRT in therapy sequence
Data on the time point of PRRT in the treatment sequence was available for 28 of the 41 patients. For approximately one third of the patients, the position of PRRT within the treatment sequence was unavailable because they had been referred from an external institution with an incomplete treatment history. Figure 4 shows that of these, 6 patients received PRRT within one year of the initial diagnosis. Of these, 2 patients received 2 further treatments within 1 year or 18 months after PRRT, and 4 patients did not receive any further treatment for at least 17 months. For 7 patients, PRRT was administered 1 year after initial diagnosis, with all 7 patients receiving another therapy within 5 months after the end of PRRT. One patient received treatment after 2 years, 2 patients after 3 years, and 4 patients after 4 years following the initial diagnosis. For 1 patient each, this period amounted to 5, 6, 10 or 13 years, and for another patient PRRT was not administered until 17 years after the initial diagnosis. All 28 patients for whom further treatment data were available after completion of PRRT, the time point of initiation for the following treatment varied between 0 and 32 months.
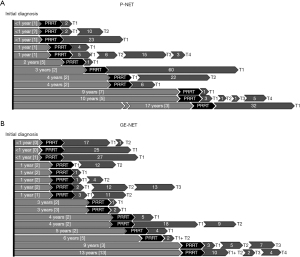
Data on the time between initial diagnosis and initiation of PRRT and the outcome was available for 35 patients. There was no significant correlation between the time between diagnosis and PRRT onset and survival (Chi-square-test, P=0.384).
Influence of previous treatment on outcome
To determine whether survival was affected by SIRT treatment prior to PRRT, patients with a previous SIRT treatment were compared to those without such a treatment. In addition, these patients were further stratified by tumor type (pancreatic tumor or non-pancreatic tumor). 41.5% of the patients had a pancreatic tumor, while in 58.5% of patients, a different tumor entity was present. 4 of the pancreatic tumors were G3 tumors.
Data on previous SIRT and survival was available for 35 of the 41 patients. 9 patients were treated with a SIRT before PRRT, of which 7 patients (78%) had died by the end of the study and 2 (22%) were still alive (Figure 5). Among patients without a previous SIRT, 14 (54%) were still alive at the end of the study, while 12 patients (46%) had died. Among patients with a previous SIRT, 5 had a pancreatic tumor and 4 another tumor entity. 4 of 5 patients with a pancreatic tumor (80%) were deceased. Contingency tables were prepared and the relative risk of mortality calculated based on previous SIRT. This analysis revealed that those patients with a SIRT prior to PRRT had a mortality risk 4,083 times higher than patients without this treatment. A subgroup analysis of patients with a previous SIRT demonstrated that the mortality risk of patients with a pancreatic tumor was 1,33 times higher than that of patients with another tumor entity who had received previous SIRT. Due to the small subgroup sample size this can merely be seen as a trend in the absence of larger-sized studies.

Symptoms of patients receiving a SIRT were compared to those who had not received SIRT. There were no significant differences in HRQoL-associated symptoms between both groups (P=0.304). Means of blood parameters did also not significantly differ between patients with and without a previous SIRT (P=0.221).
An analysis of the potential impact of the number of previous treatments on survival showed no significant correlation between the number of therapies and the outcome (Chi-square-test, P=0.281).
Influence of post-PRRT treatment on outcome
To determine whether post-SIRT SSA had an impact on the outcome, relative mortality of patients with post-PRRT SSA treatment was compared to that of patients without such a treatment. 15 of the 41 patients assessed in this study received SSA after PRRT. Of these, 9 (60%) were still alive at the end of the study, while 6 were deceased (Figure 6). In comparison, 9 of the 21 patients (39.1%) without SSA after PRRT were still alive at the end of the study, while 14 were deceased.
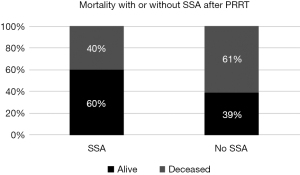
Mortality was assessed as percentage of deceased patients after completion of PRRT, for a maximum follow-up period of 248 months. The data of alive and deceased patients were then analyzed for SSA treatment after PRRT. Contingency tables and risk estimates were conducted and showed that patients without a post-PRRT SSA had a 2.33 times elevated risk of mortality. However, there was no significant correlation between SSA and mortality detectable (Mann-Whitney U test, P=0.214).
Discussion
The present study reporting real-life data of a nuclear medicine clinic within an ENETS certified center aimed to characterize the outcome of GEP-NET patients after PRRT. The first key result of this study is that PRRT altered patient symptomatic burden in GEP-NET patients. While most patients did not experience any of the analyzed HRQoL parameters before or after PRRT, more patients showed improvements in flush, loss of appetite and fatigue than those who did not. Certain parameters, particularly abdominal pain, nausea, emesis and fatigue, worsened after PRRT in some patients. The second key result of this study is that SIRT before PRRT increased mortality risk in GEP-NET patients, keeping in mind that patients receiving first a SIRT therapy might already have a more advanced or more aggressive disease than others. In several single-armed studies, the efficacy of PRRT in treating both NET (20,26-28) and other tumors, including neuroblastoma and medullary thyroid cancer, have been demonstrated. Regarding the application of this therapy for patients with GEP-NET, the first multicenter randomized controlled Phase III clinical trial to test PRRT was the NETTER-1 trial. In this study, PRRT with Lu-177-DOTATE in combination with the somatostatin analog long-acting repeatable (LAR) was shown to improve the response rate and extended progression-free survival compared to LAR alone (29). HRQoL of the participants in the NETTER-1 trial showed significant improvements in the Lu-177-DOTATE arm compared to the placebo only-arm regarding the HRQoL factors physical functioning, role functioning, global health status, fatigue, diarrhea, pain, disease-related worries and body image (25). In the NETTER-1 trial, the HRQoL parameters were evaluated as time to deterioration after randomization of the patients, whereas in the present study, patient symptomatic burden was assessed as the state before PRRT and compared to the situation after PRRT in terms of improvement, no change or no improvement.
Two currently ongoing prospective studies aim to evaluate whether PRRT may be employed as first-line treatment and potentially prove superior to other currently recommended treatment options. The NETTER-2 trial (ClinicalTrials.gov identifier NCT03972488), a multi-center, randomized, Phase III study, evaluates the efficacy and safety of PRRT as first-line treatment for patients with advanced GEP-NET. The COMPETE trial (ClinicalTrials.gov identifier NCT03049189) is a prospective, randomized, controlled, Phase III study with the aim of assessing the efficacy and safety of PRRT in comparison with targeted therapy with everolimus. These prospective trials are expected to shed light on the future role of PRRT in the treatment sequence of GEP-NET patients. Real-life data on the role of PRRT in pancreatic NET patients is scarce, and hence the present study adds to the favorable results of previous trials and is in agreement with the recommendations from European guidelines. Importantly, results obtained so far support an excellent safety profile and very low toxicity of PRRT, with concomitant beneficial effects on HRQoL even in patients with advanced NET.
Treatment options for patients with NET are constantly evolving in an attempt to stem the disease burden, particularly as the range of treatments is limited. The NETTER-1 trial was instigated to evaluate the effectiveness of the radionuclide Lu-177-DOTATATE for patients with G1 and G2 GE-NET while the NETTER-2 trial aims to evaluate treatment efficacy and safety of high grade G2 and G3 GE-NET with Lu-177-DOTATATE.
NET patients typically receive multiple sequential therapies. Up to date, there is no prospective study available that evaluates the right sequence for a GEP-NET patient. In general, GEP-NETs are a very heterogeneous group and one sequential recommendation by NET specific guidelines won’t be presented. Herein predictive biomarkers for the evaluation of the right timing of PRRT as for different systemic treatment options as well for local therapies are highly warranted. Beside the density of SSTR-2 as a target for SSA and PRRT as a predictive and prognostic marker and Ki-67 as an inverse prognostic and maybe predictive marker for PRRT and SSA no additional biomarkers in this field have been confirmed. The recently characterized blood biomarker NETest provides information about the present level of disease and its progression, therefore helping to indicate how effective PRRT is by analyzing dynamic tumor behavior via blood-based transcriptome analysis in the course of the treatment (30). This could aid in accurately identifying the tumor response to somatostatin analogs and identifying suitable therapeutic options (31). Current guidelines recommend multiple therapeutic approaches depending on the tumor entity and progression (10), yet these recommendations result from placebo-controlled trials that do not evaluate the influence of the therapy sequence on patient outcome. Only limited data from cohort studies or center analyses allows for an evaluation of the impact of the therapeutic sequence on the outcome. The results of the present study indicate a potential advantage of an early initiation of PRRT that may yield superior results compared to other treatment options such as SSA or everolimus.
Overall survival and progression-free survival were the main outcomes of PRRT trials with GEP-NET patients. Other potential advantages include the optimal time point for PRRT application within the treatment sequence, and the impact of pre-and post-PRRT therapies on the outcome of these patients in a real-life setting. The present study adds to this gap of knowledge in assessing whether SIRT before PRRT or SSA treatment after PRRT or the number of previous treatments affect the survival of GEP-NET patients. The length of the period between first diagnosis and PRRT ranged between under 1 to 17 years and had no impact on the survival of the patients for which this data was available. Of note, the time point of PRRT within the treatment sequence could be entirely determined for 28 patients and hence was missing for approximately one third of the cohort, which aggravates the evaluation of this data. The number of previous treatments did also not impact on the patients’ outcome.
Of the 38 patients for which data was available for post-PRRT therapy and survival, 15 patients had received SSA and 23 did not. Calculated odds ratios to determine whether SSA therapy might improve the prognosis of these patients suggested that the risk of dying with post-PRRT SSA therapy in our cohort was 0.429 times the risk of dying without such a therapy. In other words, patients without SSA therapy have a 2.33 times higher risk of dying compared to patients with such therapy. This is highly relevant for patients with SR-positive, well-differentiated NET as the current guideline entails an open recommendation for SSA administration after pre-treatment (32). These observed benefits of post-PRRT SSA are in line with a previous study assessing the consequences of SSA therapy as a maintenance regimen after PRRT on the outcome of GEP-NET patients. SSA as a maintenance therapy showed a clinical benefit rate for stable disease which was significantly higher than in the group without SSA maintenance therapy (33).
Therapy with SSA for GEP-NET patients with well-differentiated tumors exhibits a favorable adverse events profile in a placebo-controlled study (9) and is hence considered the first-line therapeutic choice for these patients according to the current guidelines. PRRT is recommended as second-line therapy only if SSA therapy fails or is not tolerated. Nonetheless, PRRT appears to be equally if not better tolerated and efficient than other treatment options for GEP-NET patients, including chemotherapy and targeted therapies with everolimus or tyrosine kinase inhibitors (20,27,34-36).
Concomitant SIRT with PRRT is explicitly not recommended according to the current ENETS and ESMO guidelines (37,38). SIRT may reduce the hepatic tumor load, but prospective, randomized studies assessing the advantages of combining PRRT with SIRT are missing to date. Data for SIRT and survival were available for n=35 patients in the present study, of which 9 patients had received SIRT and 26 did not. Calculation of odds ratios revealed that patients with SIRT have a 4.083 times higher risk of death than patients without SIRT.
Of the 9 patients receiving SIRT prior to PRRT, 5 had a pancreatic tumor, 4 of which were G3. The remaining 4 patients presented with a non-pancreatic tumor. Hepatic tumor load before initiation of PRRT was higher than average in 5 out of the 9 patients receiving SIRT (mean pre-PRRT hepatic tumor load 31.9%). Time between initial diagnosis of GEP-NET and initiation of PRRT for patients with prior SIRT was between 1 and 4 years with only 1 patient where PRRT was started 17 years after initial diagnosis as previous surgical debulking has been performed. Therefore, no difference in the timespan between initial diagnosis and start of PRRT in patients with SIRT and patients without SIRT could be detected (mean delay until PRRT 3.58 years). Unfortunately, the exact time point of SIRT in the treatment sequence was not available as many patients received SIRT in referring hospitals before initial assessment in our center. Follow-up for the 9 patients receiving SIRT before PRRT was between 21 and 248 months with only 2 patients (22%) alive at the end of the study. On average, patients receiving SIRT prior to PRRT did not have a longer follow-up than patients without SIRT.
Overall, the higher proportion of patients with G3 disease and an above average tumor load could be seen as prognostically detrimental for patients who received SIRT. Yet larger studies are warranted in order to further elucidate the influence of SIRT in combination with PRRT. Clinicopathologic features of tumors weren’t included in our analysis. Receiving SIRT before PRRT could be seen as a surrogate for high tumor burden and subsequently expose to a higher risk of tumor progression. Subgroup analyses for patients with and without a previous SIRT did not reveal any significant differences in the means of blood parameters or in the frequency of HRQoL symptoms. Hence, no impact of SIRT on these symptoms could be detected.
A limitation of this study is the comparatively small number of assessed patients, which aggravated subgroup analysis and stratification by tumor type and outcome. The results pertaining to the role of SIRT and SSA pre- and post-PRRT have to be interpreted with caution as the subgroups were too small to draw conclusions for the clinical practice. Nonetheless, the results are relevant with respect to current recommendations in the guidelines and need to be followed up with larger patient cohorts in the future. The second limitation is the retrospective design of this study, as only available data could be evaluated and such data collection after completion of the study is potentially prone to selection bias. HRQoL could not be assessed with a validated scoring system such as the QLQ-30 due to the retrospective design.
Conclusions
Patients with advanced GEP-NETs may benefit from PRRT with Lu-177-DOTATATE, as this treatment appears to be well tolerated and does not significantly impair the patient symptomatic burden. SIRT before PRRT seems to lower the chances of response and reduces survival. This trend was also seen if SSA was not administered after PRRT. These pilot observations must be corroborated by future prospective trials. The NETTER-2 trial employing PRRT as the first-line treatment and the COMPETE trial comparing PRRT and targeted therapies will further elucidate the advantages of PRRT for GEP-NET patients in controlled study environments.
Acknowledgments
An abstract of this study has been presented during the virtual poster session at the ASCO Annual Meeting in May 2020.
We would like to extend our special thanks to all technical and administrative personnel for their support at the Department of Nuclear Medicine at University Hospital Zurich, Switzerland. The assistance provided by the whole team was of great value and their advice and guidance made this work an inspiring experience.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-874/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-874/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-874/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-874/coif). ARS has served at advisory boards and received consulting honoraria from AMGEN, AAA, Bayer, BMS, IPSEN, Lilly, Merck, MSD, Pfizer, Roche, Sanofi, and Servier, and has received travel grants from IPSEN and ROCHE, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved and accepted by the Swiss Ethics Committee (No. BASEC-Nr. 2016-01575). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Included patients approved and signed the written General Consent form so that their specific non-coded data could be extracted from the hospital’s electronic patient register. This data was then recorded as coded and anonymous data in chart review and used for statistical analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Basu B, Sirohi B, Corrie P. Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr Relat Cancer 2010;17:R75-90. [Crossref] [PubMed]
- Pelosi G, Volante M, Papotti M, et al. Peptide receptors in neuroendocrine tumors of the lung as potential tools for radionuclide diagnosis and therapy. Q J Nucl Med Mol Imaging 2006;50:272-87. [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Oronsky B, Ma PC, Morgensztern D, et al. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017;19:991-1002. [Crossref] [PubMed]
- Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology 2004;80:3-7. [Crossref] [PubMed]
- Vijayvergia N, Dasari A. Targeted Therapies in the Management of Well-Differentiated Digestive and Lung Neuroendocrine Neoplasms. Curr Treat Options Oncol 2020;21:96. [Crossref] [PubMed]
- Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [Crossref] [PubMed]
- Stueven AK, Kayser A, Wetz C, et al. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int J Mol Sci 2019;20:3049. [Crossref] [PubMed]
- Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. [Crossref] [PubMed]
- Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:844-60. [Crossref] [PubMed]
- Chatal JF, Hoefnagel CA. Radionuclide therapy. Lancet 1999;354:931-5. [Crossref] [PubMed]
- MedGen. Lutetium (177-Lu) dotatate 2020. Available online: https://www.ncbi.nlm.nih.gov/medgen/473977.
- Kam BL, Teunissen JJ, Krenning EP, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2012;39:S103-12. [Crossref] [PubMed]
- Available online: http://www.lnhb.fr/nuclides/Lu-177_tables.pdf
- de Jong M, Breeman WA, Valkema R, et al. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med 2005;46:13S-7S. [PubMed]
- Reubi JC, Waser B, Schaer JC, et al. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 2001;28:836-46. [Crossref] [PubMed]
- Akhurst T. Nuclear medicine techniques in hepatobiliary and pancreatic disease. Elsevier: 2012:241-71.e3.
- Krenning EP, Kooij PP, Bakker WH, et al. Radiotherapy with a radiolabeled somatostatin analogue, 111In-DTPA-D-Phe1-octreotide. A case history. Ann N Y Acad Sci 1994;733:496-506. [Crossref] [PubMed]
- Krenning EP, de Jong M, Kooij PP, et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol 1999;10:S23-9. [Crossref] [PubMed]
- Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124-30. [Crossref] [PubMed]
- mhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416-23.
- Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 2011;38:2125-35. [Crossref] [PubMed]
- Poeppel TD, Boy C, Bockisch A, et al. Peptide receptor radionuclide therapy for patients with somatostatin receptor expressing tumours. German Guideline (S1). Nuklearmedizin 2015;54:1-N2. [PubMed]
- Kwekkeboom DJ, de Herder WW, van Eijck CH, et al. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 2010;40:78-88. [Crossref] [PubMed]
- Strosberg JR, Wolin EM, Chasen BA, et al. First update on overall survival, progression-free survival, and health-related time-to-deterioration quality of life from the NETTER-1 study: 177Lu-Dotatate vs. high dose octreotide in progressive midgut neuroendocrine tumors. J Clin Oncol 2018;36:4099. [Crossref]
- Bodei L, Kwekkeboom DJ, Kidd M, et al. Radiolabeled Somatostatin Analogue Therapy Of Gastroenteropancreatic Cancer. Semin Nucl Med 2016;46:225-38. [Crossref] [PubMed]
- Ezziddin S, Khalaf F, Vanezi M, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014;41:925-33. [Crossref] [PubMed]
- Delpassand ES, Samarghandi A, Zamanian S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas 2014;43:518-25. [Crossref] [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer 2015;22:561-75. [Crossref] [PubMed]
- Modlin IM, Kidd M, Malczewska A, et al. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinol Metab Clin North Am 2018;47:485-504. [Crossref] [PubMed]
- Deutsche Gesellschaft für Gastroenterologie. Practice guideline neuroendocrine tumors - AWMF-Reg. 021-27. Z Gastroenterol 2018;56:583-681. [Crossref] [PubMed]
- Yordanova A, Wicharz MM, Mayer K, et al. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin Cancer Res 2018;24:4672-9. [Crossref] [PubMed]
- Sansovini M, Severi S, Ianniello A, et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with (177)Lu-D OTATATE. Eur J Nucl Med Mol Imaging 2017;44:490-9. [Crossref] [PubMed]
- Brabander T, van der Zwan WA, Teunissen JJM, et al. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 2017;23:4617-24. [Crossref] [PubMed]
- Demirci E, Kabasakal L, Toklu T, et al. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours including high-grade (WHO G3) neuroendocrine tumours: response to treatment and long-term survival update. Nucl Med Commun 2018;39:789-96. [Crossref] [PubMed]
-
ENETS-G. ENETS Guidelines & Standards of Care - ESMO. ESO Guidelines Available online: https://www.esmo.org/guidelines/


