
Pre-operative chemoradiotherapy with or without induction chemotherapy for operable locally-advanced esophageal cancer
Highlight box
Key findings
• Induction chemotherapy prior to chemoradiation, compared with chemoradiation alone, was not associated with improved distant metastasis, overall or progression-free survival for patients with operable locally advanced esophageal cancer.
What is known and what is new?
• Neoadjuvant chemoradiation followed by esophagectomy is a standard treatment option.
• A phase II trial, which compared induction carboplatin and paclitaxel versus 5-fluorouracil and oxaliplatin (FOLFOX) with concurrent chemotherapy during radiotherapy tailored per interval response, suggested favorable outcomes amongst those who responded to FOLFOX and received FOLFOX-based chemoradiation.
• The current study suggests that induction chemotherapy prior to pre-operative chemoradiation is not associated with improved progression-free or overall survival
What is the implication, and what should change now?
• Pre-operative chemoradiation remains a standard option for patients with operable, locally advanced esophagus cancer. Further research is needed to assist in patient selection and optimization of the peri-operative regimen to improve outcomes.
Introduction
The preferred treatment strategy for patients with operable, locally advanced thoracic esophagus or gastroesophageal junction (GEJ) cancer is preoperative chemoradiotherapy (CRT) followed by esophagectomy (1-5). This regimen is most strongly supported by the ChemoRadiotherapy for Esophageal cancer followed by Surgery Study (CROSS) which randomized patients to esophagectomy alone vs. pre-operative CRT and demonstrated improvements in margin-negative (R0) resection rate, locoregional control, and overall survival (OS) with the addition of CRT (6). Despite these improvements, approximately 30–50% of patients experience distant metastasis and 50–60% of patients ultimately die from the disease (7).
There has been evolving interest in intensifying pre-operative therapy using a regimen of induction chemotherapy followed by CRT (IC-CRT) with the goal of improving outcomes (8-10). Unfortunately, past investigations of IC-CRT vs. CRT alone have not demonstrated significant improvements in progression-free (PFS) or OS (9-11).
Following initial presentation of CALGB 80803 (12) our institutional practice patterns shifted towards treating patients with a 5-fluorouracil (5-FU), leucovorin, and oxaliplatin (FOLFOX)-based IC-CRT strategy. As there has not yet been level I evidence suggesting that IC-CRT improves PFS or OS when compared to CRT, we sought to compare PFS and OS in patients with operable, locally advanced esophagus or GEJ cancer (LA-EC) treated with pre-operative intent IC-CRT vs. CRT. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1005/rc).
Methods
Patient cohort
We performed a single instutition retrospective cohort study including patients who had received CRT for LA-EC between 2013–2019 within a single institutional care network. Patients were included if they had primary esophagus or GEJ cancer, were deemed operable candidates, and received pre-operative intent CRT. Patients were excluded if they had metastatic disease at diagnosis, received palliative intent radiotherapy (<40 Gy), or had less than 1 month follow-up after completion of CRT. Eligible patients were divided into two groups, IC-CRT (one or more cycles of induction chemotherapy, administered prior to CRT), and CRT. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the institutional review board of Yale University (IRB No. 2000027091). Given the restrospective nature of the study, consent was not required.
Patient interventions and assessments
Patients were staged with positron emission tomography-computed tomography (PET-CT) and received either neoadjuvant CRT or IC-CRT, which included at least one cycle of induction chemotherapy followed by CRT. Re-staging occurred after completion of neoadjuvant therapy at which time esophagectomy was performed for those eligible. Following completion of therapy, patients were typically evaluated in follow up approximately every 3–6 months per routine clinical care. Follow-up examinations included physical examination, hematological and biochemical testing, and diagnostic imaging or endoscopy as deemed clinically indicated. Local recurrence was defined as recurrence or persistence at the primary tumor site; regional recurrence sites were defined as regional lymph nodes; distant recurrence sites were defined as non-regional lymph nodes or distant organs. Recurrences were diagnosed by histologic confirmation or, when biopsy was not available, clinically per radiographic assessment.
Endpoints
The co-primary endpoints of the study were PFS and OS between IC-CRT and CRT treatment cohorts. Survival outcomes were secondarily assessed in subsets of patients based on histology. Additional analyses were performed for those with adenocarcinoma that were treated with at least 3 cycles of induction FOLFOX (8), and those with adenocarcinoma histology who underwent successful curative-intent esophagectomy, as these were the subgroups hypothesized to most likely derive long-term benefit from an IC-CRT strategy (8,10). Secondary endpoints included clinical and treatment characteristics associated with PFS and OS, pathologic response, and patterns of disease recurrence. We report surrogate measures of dysphagia and nutrition descriptively, including need for feeding tube placement during therapy, percentage of baseline weight loss, and change in serum albumin.
Statistical analysis
Baseline characteristics of the two cohorts were compared using chi-squared and Wilcoxon rank-sum test. OS and PFS were defined from the time of completion of CRT and were estimated using the Kaplan-Meier (KM) method. Patients lost to follow-up were censored at time of last encounter. Comparisons between treatment cohorts were made using the log-rank test. Univariable Cox proportion hazards regression was used to assess for associations between baseline patient and treatment characteristics and OS. Multivariable analysis (MVA), including histology, clinical T stage, lymph node involvement, Karnofsky performance status (KPS), grade, and sex (9,10,13) along with treatment group was also performed.
Chi-squared analysis was performed to assess impact of treatment group on pathologic outcomes. The cumulative incidence of local recurrence (LR), locoregional recurrence (LRR), and distant metastasis (DM) are reported. Surrogate of dysphagia and nutritional status are reported descriptively. A two-sided P value of <0.05 was considered statistically significant. Statistical analysis was performed using STATA version 13.3.
Results
A total of 161 patients with LA-EC were treated with CRT between 2013-2019 at our institution, of which, 95 were deemed surgical candidates at diagnosis and were included in this analysis. Baseline patient and treatment characteristics are demonstrated in Tables 1,2, respectively.
Table 1
| Characteristics | IC-CRT (n=59) | CRT (n=36) | P value |
|---|---|---|---|
| Age, years | 0.46 | ||
| ≤60 | 12 [20] | 7 [19] | |
| 61–70 | 31 [53] | 15 [42] | |
| >70 | 16 [27] | 14 [39] | |
| Sex | 0.81 | ||
| Male | 48 [81] | 30 [83] | |
| Female | 11 [19] | 6 [17] | |
| Race | 0.81 | ||
| White | 55 [93] | 34 [94] | |
| AA/others | 4 [7] | 2 [6] | |
| Smoking | 0.76 | ||
| Never | 17 [29] | 8 [22] | |
| Former | 32 [54] | 22 [61] | |
| Current | 10 [17] | 6 [17] | |
| Alcohol abuse | 0.75 | ||
| No | 52 [90] | 33 [92] | |
| Yes | 6 [10] | 3 [8] | |
| BMI, kg/m2 | 0.33 | ||
| <18.5 | 0 | 1 [3] | |
| 18.5–24.9 | 21 [36] | 7 [19] | |
| 25–29.9 | 15 [25] | 13 [36] | |
| >30 | 23 [39] | 15 [42] | |
| Location | 0.28 | ||
| Lower thoracic/GEJ | 53 [90] | 32 [89] | |
| Mid thoracic | 3 [5] | 3 [8] | |
| Upper thoracic | 3 [5] | 0 | |
| Cervical | 0 | 1 [3] | |
| Histology | 0.09 | ||
| Adenocarcinoma | 51 [86] | 26 [72] | |
| Squamous cell carcinoma | 8 [14] | 10 [28] | |
| Grade | 0.30 | ||
| 1 | 0 [0] | 2 [6] | |
| 2 | 24 [41] | 12 [33] | |
| 3 | 28 [47] | 17 [47] | |
| Unknown | 7 [12] | 5 [14] | |
| T stage | 0.53 | ||
| 1 | 2 [3] | 3 [8] | |
| 2 | 8 [14] | 6 [17] | |
| 3 | 48 [83] | 27 [75] | |
| 4 | 1 [2] | 0 | |
| N stage | 0.76 | ||
| 0 | 15 [25] | 7 [19] | |
| 1 | 24 [41] | 17 [47] | |
| 2 | 19 [32] | 12 [33] | |
| 3 | 1 [2] | 0 [0] | |
| HER2 | 0.78 | ||
| Negative | 31 [58] | 16 [57] | |
| Positive | 12 [23] | 8 [29] | |
| Unknown | 10 [19] | 4 [14] | |
| Baseline weight loss (%) | 0.12 | ||
| None | 7 [12] | 5 [14] | |
| 0–10% | 23 [39] | 22 [61] | |
| 10–20% | 24 [41] | 8 [22] | |
| 20–30% | 5 [8] | 1 [3] | |
| KPS (median=80) | 0.62 | ||
| ≤80 | 31 [53] | 17 [47] | |
| >80 | 28 [47] | 19 [53] | |
| CCI (median =5) | 0.70 | ||
| ≤5 | 21 [36] | 15 [42] | |
| >5 | 38 [64] | 21 [58] | |
| Feeding tube prior to treatment | 0.53 | ||
| No | 56 [95] | 33 [92] | |
| Yes | 3 [5] | 3 [8] | |
| Albumin at diagnosis (median = 4.1) | 0.09 | ||
| ≤ median | 27 [46] | 23 [64] | |
| > median | 32 [54] | 13 [36] | |
| Year of treatment | 0.01 | ||
| 2012–2013 | 12 [20] | 7 [19] | |
| 2014–2015 | 9 [15] | 16 [44] | |
| 2016–2017 | 26 [44] | 10 [28] | |
| 2018–2019 | 12 [20] | 3 [8] |
P values generated using chi-squared test. “Other” Race includes unknown race in 2 patients. There was 1 Hispanic patient in the CRT group. IC-CRT, induction chemotherapy and chemoradiation; CRT, chemoradiation; BMI, body mass index; KPS, Karnofsky performance status; CCI, Charlson comorbidity index.
Table 2
| Characteristics | IC-CRT (n=59) | CRT (n=36) | P value |
|---|---|---|---|
| Induction chemotherapy regimen | |||
| Platinum + taxane | 12 [20] | NA | |
| 5FU + platinum | 45 [76] | NA | |
| Others | 2 [3] | NA | |
| No. cycles induction chemotherapy, median (IQR) | 3 [2–3] | NA | |
| Concurrent chemotherapy regimen | <0.001 | ||
| Platinum + taxane | 17 [29] | 34 [94] | |
| 5FU + platinum | 40 [68] | 2 [6] | |
| Others | 2 [3] | 0 | |
| Post-operative systemic therapy | 0.12 | ||
| No | 58 [98] | 33 [92] | |
| Yes | 1 [2] | 3 [8] | |
| Delivered RT dose (Gy), median [IQR]* | 50 [50–50.4] | 50 [50–50.4] | 0.87 |
| Number of RT fractions, median [IQR]* | 25 [25–28] | 25 [25–28] | 0.99 |
| Esophagectomy completed | 31 [56] | 24 [44] | 0.18 |
P values generated using chi-square except for those with (*) which were compared using Wilcoxon-rank sum test. IC-CRT, induction chemotherapy and chemoradiation; CRT, chemoradiation; IQR, interquartile range; RT, radiation therapy; 5FU, 5 fluorouracil.
Survival
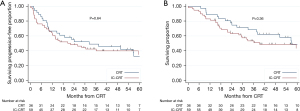
The median patient follow-up of the overall cohort was 37.7 months (IQR: 16.8–56.1 months): 29.6 months (IQR: 15.2–47.8) for IC-CRT and 42 months (IQR: 23.5–60) for CRT. The estimated 3-year PFS and OS for the total cohort were 44% (95% CI: 33–53%) and 58% (95% CI: 47–67%), respectively. The median PFS and OS for the IC-CRT and CRT cohorts were 22.0 months (95% CI: 12.0–59.3) vs. 32.3 months (95% CI: 10.1–56.9) (P=0.64) and 39.3 months (95% CI: 23.2–not reached) vs. 56.9 months (95% CI: 37.7–not reached) (P=0.36), respectively. KM estimates of PFS and OS are demonstrated in Figure 1. There was no difference when comparing IC-CRT vs. CRT in the subset of patients who underwent esophagectomy (Figure S1).
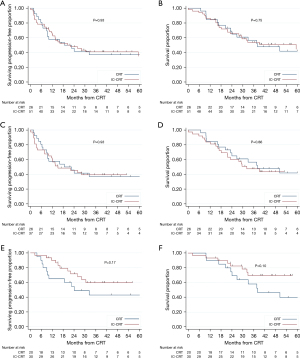
When restricting the analysis to patients with adenocarcinoma histology (AC-EC, n=77), the median PFS and OS for the IC-CRT vs. CRT cohorts were 18.7 months (95% CI: 9.0–not reached) vs. 22.0 months (95% CI: 12.0–not reached) (P=0.93) and 38.1 months (95% CI: 23.4–not reached) vs. 59.4 months (95% CI: 27.9–not reached) (P=0.75), respectively (Figure 2A,2B). Similarly, when comparing patients with AC-EC who received IC-CRT with ≥3 cycles of induction FOLFOX (8) vs. CRT, there was no difference in median PFS (15.1 vs. 18.7 months, P=0.93) or median OS (32.3 vs. 38.1 months, P=0.86) (Figure 2C,2D). In the subset of AC-EC patients who had esophagectomy (n=50), the median survival was not met in IC-CRT cohort however there was no statistical difference in outcome by log-rank in PFS or OS with 3-year PFS of 60% (95% CI: 39–75%) vs. 43% (95% CI: 21–63%) and 3-year OS of 70% (95% CI: 48–84%) vs. 58% (95% CI: 33–77%) (Figure 2E,2F). Lastly, when restricting analysis further to AC-EC receiving at least 3 cycles of induction FOLFOX, CRT, and esophagectomy versus CRT and esophagectomy (n=39) there was still no significant difference in outcome between IC-CRT and CRT cohorts (Figure S2).
Among patients with squamous cell carcinoma (SCC) (n=18), the PFS numerically favored CRT though did not reach statistical significance (6.4 vs. 56.6 months, P=0.28), however, OS was significantly shorter for those in the IC-CRT vs. CRT cohorts (17.4 vs. 63.0 months, P=0.01) (Figure S3).
On UVA, T3-4 (vs. T1-2) disease was associated with poorer PFS [4.45 (1.61, 12.30), P=0.004] while KPS >80 was associated with improved OS [0.53 (0.30, 0.95), P=0.03]. No other variables were associated with survival outcomes (Table S1). On MVA using variables supported in the literature and treatment group, T3-4 disease was associated with poorer PFS [4.94 (1.76, 13.86), P=0.002], and higher KPS at diagnosis was associated with improved OS [0.47 (0.25, 0.87), P=0.02]. Receipt IC-CRT, compared with CRT, was not associated with improved PFS or OS on either UVA (Table S1) or MVA (Table 3).
Table 3
| Characteristics | PFS, median [IQR] | OS, median [IQR] |
|---|---|---|
| Induction chemotherapy yes (ref = no) | 1.07 [0.60, 1.90] | 1.14 [0.59, 2.18] |
| Male sex (ref = female) | 0.66 [0.30, 1.45] | 0.90 [0.39, 2.05] |
| Adenocarcinoma (ref = SCC) | 1.08 [0.50, 2.32] | 1.36 [0.57, 3.26] |
| Grade 3 (ref =1 or 2) | 0.66 [0.36, 1.19] | 0.74 [0.39, 1.41] |
| Grade unknown | 1.24 [0.54, 2.83] | 0.88 [0.34, 1.41] |
| T stage 3 or 4 (ref = 1 or 2) | 4.94 [1.76, 13.86] | 3.30 [1.16, 9.37] |
| N1-3 (ref =N0) | 1.44 [0.72, 2.88] | 1.32 [0.62, 2.82] |
| KPS >80 (ref ≤80) | 0.64 [0.37, 1.11] | 0.47 [0.25, 0.87] |
SCC, squamous cell carcinoma; KPS, Karnofsky performance status; PFS, progression-free; OS, overall survival.
Pathologic outcomes
Amongst the 55 patients who underwent esophagectomy, median interval from completion of RT to surgery was 63 days (IQR: 49.5–82). The rate of pCR was 48% vs. 29% (P=0.15) for the IC-CRT vs. CRT cohorts (Table S2). When comparing patients with adenocarcinoma histology who received IC-CRT with ≥3 cycles of induction FOLFOX vs. CRT, pCR rates were 58% vs. 20%, P=0.02.
Patterns of recurrence
The patterns of recurrence are demonstrated in Table S3. Of the 95 total patients, 45 (47%) experienced disease recurrence. The proportion of patients who recurred were similar between the IC-CRT (53%) and CRT (50%) treatment cohorts, P=0.81, as was the median time to disease recurrence: 9.5 months (IQR: 2.2–14.0) and 9.5 months (IQR: 5.8–18.8). The cumulative incidences of local recurrence, regional recurrence, and distant metastasis were 17%, 15%, and 44%. The site of first recurrence was most commonly distant only (32% for IC-CRT and 31% for CRT), while only 3% and 8% experienced local-only recurrence, respectively. When analyzing the cN+ subset (n=73), the rates for DM remained similar at 48% for the CRT cohort and 43.5% for the IC-CRT cohort, P=0.67.
Neoadjuvant treatment toxicity
Surrogates of nutritional and dysphagia status are demonstrated in Table 4. The median weight loss from time of diagnosis to completion of neoadjuvant therapy was 13.2% (IQR: 6.1–24.6%). For the IC-CRT vs. CRT cohorts, the median weight loss was 18.5% (IQR: 7.9–28.7%) and 9.7% (4.9–17.3%), respectively. Feeding tubes were placed in 24% of patients in the IC-CRT cohort and 6% of the CRT cohort. The median change in serum albumin for the IC-CRT and CRT cohorts were +0.4 (IQR: 0.1–0.7) and +0.4 (IQR: 0–0.8).
Table 4
| Outcome | IC-CRT | CRT |
|---|---|---|
| Feeding tube required during CRT, n [%] | 14 [24] | 2 [6] |
| Any type feeding intervention*, n [%] | 17 [29] | 6 [17] |
| % baseline weight loss during therapy, median [IQR] | 13.7 [7.4–21.3] | 8.95 [3.9–13.2] |
| % diagnosis weight loss during therapy, median [IQR] | 18.5 [17.9–28.7] | 9.7 [4.9–17.3] |
| Albumin change during therapy, median [IQR] | 0.40 [0.1–0.7] | 0.40 [0–0.8] |
*, Any intervention includes feeding tube and/or stent placement. IC-CRT, induction chemotherapy and chemoradiation; CRT, chemoradiation.
Treatment breaks occurred in 26 (44%) and 13 (36%), P=0.44, patients in the IC-CRT and CRT cohorts, respectively. The length of treatment break was similar in each cohort at a median 3 days (IQR: 1–6), P=0.81. Nineteen patients (32%) were hospitalized during IC-CRT compared to 8 (22%) during CRT (P=0.30). There was one post-operative/peri-operative death in our cohort as a result of anastomotic leak; surgical complications did not differ between the cohorts and are detailed in Table S4.
Discussion
We report a single institution retrospective cohort study evaluating patients with locally advanced, operable, thoracic esophagus or GEJ cancer treated with pre-operative IC-CRT versus CRT. The majority of patients in the respective treatment cohorts were treated with induction FOLFOX followed by FOLFOX-RT (8) or a carboplatin plus paclitaxel-based CRT (1,14) treatment regimen. We did not identify significant improvements in PFS or OS with the use of IC-CRT compared to CRT alone in the overall cohort nor did we when restricting analyses to subgroups of patients we hypothesized would derive greatest benefit from an IC-CRT strategy—patients with adenocarcinoma histology, adenocarcinoma histology treated with ≥3 cycles of induction FOLFOX, as was done in CALGB 80803 (8), or those with adenocarcinoma histology who underwent esophagectomy. Moreover, IC-CRT was not associated with improved PFS or OS on univariate or multivariate analysis. Similarly, there were no differences in the patterns of disease recurrence between treatment cohorts, with the majority of patients experiencing distant metastasis.
The currently preferred treatment strategy for patients with operable, locally advanced thoracic esophagus or GEJ cancer is preoperative CRT followed by esophagectomy (1-5). This approach is most strongly supported by the CROSS Trial which demonstrated a pCR rate of 29% and improvements in R0 resection rates, locoregional control, and OS with the addition of pre-operative CRT (6). Improvements in surgical technique and the inclusion of pre-operative CRT has resulted in a significant reduction in locoregional recurrence, however the competing risk of distant metastasis remains high and is often the driver of morbidity and mortality for patients (1,7,15). This has prompted investigation of systemic treatment intensification with the goal of reducing rates of distant metastasis and improving survival (8-10,16).
Multiple investigators have studied the addition of induction chemotherapy to pre-operative CRT. Ajani et al. performed a randomized phase II trial comparing induction FOLFOX followed by FOLFOX-RT vs. concurrent FOLFOX-RT in a cohort of patients with predominately adenocarcinoma histology (97%) (9). They did not demonstrate any improvement in pCR (26% vs. 13%, P=0.094) or median OS (44 vs. 46 months, P=0.69) with the use of IC-CRT. The Alliance/NCCTG N0849 phase II trial randomized patients with esophagus or GEJ adenocarcinoma to induction docetaxel, oxaliplatin, capecitabine followed by 5FU and oxaliplatin-based CRT vs. CRT alone. Of the 55 evaluable patients, the pCR rates (primary endpoint) for the IC-CRT and CRT cohorts were 28.6% and 40.7% (P=0.34), which led to trial closure at interim analysis due to perceived futility. There was no improvement in OS (HR 0.70, 95% CI: 0.35–1.40) for the overall cohort. However, they identified a suggestion of improvement in 3-year OS (57.1% vs. 41.7%) in the IC-CRT cohort, which upon post-hoc exploratory analysis seemed to be driven by improved PFS in the sub-group of patients who underwent R0 resection (HR 0.40, 95% CI: 0.18–0.91, P=0.023) and improved OS in patients with well or moderately differentiated tumors (HR 0.33, 95% CI: 0.11–1.02, P=0.042). Similarly, Yoon et al. randomized patients with predominately SCC histology (98%) to S-1 plus oxaliplatin based-CRT with or without S-1 plus oxaliplatin induction chemotherapy (11). They did not demonstrate any improvement in pCR (23.4% vs. 38%), 2-year PFS (58.4% vs. 58.6%), or 2-year OS (60.7% vs. 63.7%) with the use of IC-CRT vs. CRT, respectively.
Recent interest in IC-CRT has been driven by the promising outcomes from the CALGB 80803 trial (8). In this trial, patients were randomized to receive induction FOLFOX or carboplatin and paclitaxel. Following interim PET-CT, patients with responsive disease continued the same concurrent chemotherapy regimen during CRT where-as those with non-responsive disease crossed over to the alternate chemotherapy regimen. The pCR rates were 40% for FOLFOX responders and 18-20% for the FOLFOX and carboplatin plus paclitaxel non-responders who crossed over to the alternative regimen- each of which was numerically higher than the 14% pCR rate in the carboplatin plus paclitaxel responder sub-group. Patients who received induction FOLFOX followed by FOLFOX-RT achieved a 5-year OS of 53%, thus suggesting FOLFOX may be a particularly effective chemotherapy regimen when utilizing an induction chemotherapy strategy.
Following presentation of CALGB 80803, our institutional practice shifted from a predominately CROSS trial based preoperative CRT strategy (platinum/taxane doublet with 50 Gy/25 fractions) to more routine utilization of a CALGB 80803 FOLFOX-based IC-CRT followed by FOLFOX-RT strategy (8) (Table 1) without routine use of PET-adaptation given apparently superior outcomes with FOLFOX regardless of PET response. However, since CALGB 80803 did not directly compare IC-CRT vs. CRT and improved PFS or OS have not yet been demonstrated with IC-CRT when compared with CRT, we aimed to investigate whether the addition of induction chemotherapy improved outcomes for our patients. Additionally, we explored specific patient subsets who we hypothesized would derive greatest benefit from an IC-CRT strategy based upon the aforementioned randomized trial data (8,10).
In the overall cohort, there was a numerically higher, yet non-significant, difference in pCR with use of IC-CRT vs. CRT, 48% vs. 29% (P=0.15). In the subset of patients with AC-EC histology who received ≥3 cycles of induction FOLFOX IC-CRT vs. CRT, the pCR rate was improved, 58% vs. 20%, P=0.02, comparing favorably to the CALGB FOLFOX-responders despite absence of PET-adaptation. However, despite these improvements in pCR, we did not detect any improvements in PFS or OS with IC-CRT vs. CRT alone by log-rank testing or when using multivariable cox regression. To approximate the IC-CRT CALGB 80803 intervention, an exploratory analysis was performed assessing only AC-EC patients treated with at least 3 cycles of induction 5FU+platinum compared to those receiving CRT alone, yet there was still no improvement in PFS or OS. Lastly, we looked at patients who completed pre-operative therapy and underwent esophagectomy, as the NCCTG N0849 trial suggested a 3-year PFS benefit with IC-CRT compared with CRT in patients who underwent margin-negative resection. Yet in our series, we still did not detect any significant differences in outcomes. Furthermore, patterns of disease recurrence did not differ between patients treated with IC-CRT vs. CRT alone, with the majority of recurrences occurring at distant sites at a median of 9.5 months. We do acknowledge that our study follow-up is short and that greater differences, particularly in the subset of patients with adenocarcinoma histology who underwent esophagectomy, may arise with longer follow-up. However, based upon our study and the aforementioned trial data we cannot recommend routine use of IC-CRT in patients with AC-EC. Further exploration into selection of patients for IC-CRT, the optimal chemotherapy agent(s), incorporation of immunotherapy either in the neoadjuvant (17) or adjuvant (16) setting, and sequencing of therapy for these patients is necessary to improve outcomes for patients.
IC-CRT is not routinely utilized in patients with SCC of the esophagus, as in many other disease sites, the use induction chemotherapy and prolongation of the overall treatment time (OTT) has been detrimental to OS (18-20). We hypothesize the prolonged OTT (approximately 6 weeks for CRT versus 12 weeks for IC-CRT) affects SCC more significantly than AC-EC and is one of the reasons for the stark difference in outcomes between the two histologies. While our analysis is underpowered to answer this question and there may be considerable indication biases with patients with more advanced SCC receiving an IC-CRT strategy, our data do continue to suggest that CRT alone is the preferred pre-operative treatment strategy for patients with SCC when feasible.
An important aspect of trimodality treatment for patients wth LA-EC is nutrition, as they must have adequate reserve and KPS to withstand therapy, and recover from, an esophagectomy. In this cohort of patients, only 6% of patients required a feeding tube during their course of CRT compared to 24% in the IC-CRT cohort. Patients receiving IC-CRT lost twice as much weight during neoadjuvant treatment as those receiving CRT. There is an institutional bias to offer IC-CRT as cytoreduction strategy for patients with subjective report of baseline dysphagia. However, between treatment cohorts, we did not appreciate any differences in T or N stage, percentage of baseline weight loss, indication for feeding at diagnosis, or baseline albumin at time of diagnosis when treatment decisions were being made.
While the overall cohort of patients included in this analysis had technically resectable disease at diagnosis, 42% did not ultimately proceed to esophagectomy for a variety of reasons listed in the appendix. The majority of patients either declined surgery by the time CRT +/− induction was finished, had interval progression of disease which precluded an operation, or their performance status was such that they were no longer eligible. This demonstrates the toll that multimodal treatment may have on this population of patients.
Our analysis is limited by its single institution and retrospective nature resulting in an inability to control for uncaptured clinical or pathologic features that may have impacted treatment decisions. Additionally, our follow-up is relatively short and it is possible that further differences may manifest in longer-term follow-up. Our study is limited by small sample size, however it represents a relatively large single institution analysis which incorporates extensive patient-level data to help analyze this clinical question. All patients analyzed in this cohort were initially considered surgical candidates, however, 58% ultimately underwent esophagectomy which is lower than the rates of 74–81% reported in NCCTG N0849 (10) and CALGB 80803 trials (8). Notably, we do not routinely collect prospective objective measurements of dysphagia severity and therefore the interactions of preoperative therapy regimen, pre-treatment dysphagia, and risk of needing a feeding tube cannot be thoroughly assessed.
Conclusions
In conclusion, in our retrospective series assessing the outcomes of patients with operable LA-EC, the addition of induction chemotherapy prior to preoperative chemoradiotherapy was not associated with improved OS or PFS. Despite excellent locoregional control with this approach, the predominant pattern of recurrence remains to be at distant sites. Further work is needed to optimize the treatment strategy to improve outcomes for patients with LA-EC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1005/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1005/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1005/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1005/coif). GWP reports RSNA/AUR/APDR/SCARD Radiology Education Research Development Grant for medical education. JL serves as an Consultant for Merck, AstraZeneca, Novartis, Deciphera, Opsen Pharma, and Celgene. MC reports Grant funding from the National Cancer Institute, and personal fees from Agios Pharmaceuticals, Eisai Inc, and AstraZeneca. SS serves as an Consultant for Genentech, QED Therapeutics, Exelixis, Astrazeneca, and Merck. KJ serves as an Editor for Radoncquestions.com, LLC. None of the above disclosures directly pertain to this work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the institutional review board of Yale University (IRB No. 2000027091). Given the restrospective nature of the study, consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer 2017;81:183-90. [Crossref] [PubMed]
- NCCN. Esophageal and Esophagogastric Junction Cancers (Version 4.2021). Sept 20, 2021); Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Goodman KA, Ou FS, Hall NC, et al. Randomized Phase II Study of PET Response-Adapted Combined Modality Therapy for Esophageal Cancer: Mature Results of the CALGB 80803 (Alliance) Trial. J Clin Oncol 2021;39:2803-15. [Crossref] [PubMed]
- Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2013;24:2844-9. [Crossref] [PubMed]
- Yoon HH, Ou FS, Soori GS, et al. Induction versus no induction chemotherapy before neoadjuvant chemoradiotherapy and surgery in oesophageal adenocarcinoma: a multicentre randomised phase II trial (NCCTG N0849 (Alliance)). Eur J Cancer 2021;150:214-23. [Crossref] [PubMed]
- Yoon DH, Jang G, Kim JH, et al. Randomized phase 2 trial of S1 and oxaliplatin-based chemoradiotherapy with or without induction chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2015;91:489-96. [Crossref] [PubMed]
- Goodman KA, Niedzwiecki D, Hall N, et al. Initial results of CALGB 80803 (Alliance): A randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J Clin Oncol 2017;35:1. [Crossref]
- Lin SH, Hobbs BP, Verma V, et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J Clin Oncol 2020;38:1569-79. [Crossref] [PubMed]
- Safran HP, Winter K, Ilson DH, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): a multicentre, randomised, phase 3 trial. Lancet Oncol 2022;23:259-69. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- National Cancer Institute. Pembrolizumab, Combination Chemotherapy, and Radiation Therapy Before Surgery in Treating Adult Patients With Locally Advanced Gastroesophageal Junction or Gastric Cardia Cancer That Can Be Removed by Surgery. (cited 2021 Oct. 14); Available online: https://clinicaltrials.gov/ct2/show/NCT02730546.
- Shaikh T, Handorf EA, Murphy CT, et al. The Impact of Radiation Treatment Time on Survival in Patients With Head and Neck Cancer. Int J Radiat Oncol Biol Phys 2016;96:967-75. [Crossref] [PubMed]
- Ben-Josef E, Moughan J, Ajani JA, et al. Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of Radiation Therapy Oncology Group trials 87-04 and 98-11. J Clin Oncol 2010;28:5061-6. [Crossref] [PubMed]
- Tanderup K, Fokdal LU, Sturdza A, et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol 2016;120:441-6. [Crossref] [PubMed]


