
From KIT-mutated into wild-type: dedifferentiation of gastrointestinal stromal tumor in adolescent patient—a case report
Highlight box
Key findings
• This case exhibits dedifferentiation of GIST (from KIT-mutated into wild-type) and describes challenges in its proper treatment and follow-up.
What is known and what is new?
• GISTs are extremely rare in pediatric population and their management must differ, basing on the biology and molecular profile of the disease.
• Non-standard diagnostic techniques and extended molecular profiling might be useful in the treatment and subsequent follow-up of pediatric GISTs.
What is the implication, and what should change now?
• Unified guidelines concerning the management of gastrointestinal stromal tumors in pediatric population should be created, bearing in mind their pediatric and adult type.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal proliferation arising from interstitial Cajal cells of the gastrointestinal (GI) tract. This type of tumor is predominantly found in elderly adults, and such diagnosis in the pediatric population tends to be an exceptional phenomenon. Less than 2% of GISTs are diagnosed in children, giving 0.02 children/million/year (1,2).
Most GISTs are sporadic and solitary tumors with receptor kinase tyrosine (KIT) or platelet-derived growth factor receptor α (PDGFRA) mutations (3). However, in children, the tumors tend to be wild-type or possess Insulin-Like Growth Factor 1 Receptor (IGF1R) or succinate dehydrogenase (SDH) complex (gene abnormalities (4). They are found predominantly in females in the stomach, though the tumor may be located anywhere in the GI tract and other organs, e.g., liver the most frequent localization of GIST metastases), lymph nodes, peritoneum, omentum, abdominal wall, mesentery, rarely lungs, skin or subcutaneous tissue (5). Pediatric GISTs have different histology, epithelioid or mixed, compared to the spindle cell adult histology (4,6). They also metastasize more often but more indolently (4).
The best care for patients with suspicion and diagnosis of GIST is a biopsy followed by R0 surgical excision. The surgical approach must be cautious as the tumors tend to disintegrate or bleed, which is a risk factor for recurrences. Furthermore, the surrounding lymph nodes are not routinely removed (7). Besides histopathological examination, genetic tests should be executed to confirm whether the tumor harbors any of the most common mutations, their type and location. Imatinib treatment may be introduced in case of tumor rupture, incomplete tumor resection or disease progression when there is predicted sensitivity to this agent (i.e., exon 9 or 11 KIT mutations) (8). If the child is diagnosed with a pediatric GIST, the algorithm shall be different, and pediatric GIST guidelines should be followed (9).
Herein, we report a case of a 10-year-old female patient diagnosed with a GIST of the stomach with subsequent regional spread, primarily KIT-mutated (exons 13 and 17). She is now after the second total resection of the tumor. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1111/rc).
Case presentation
A 10-year-old female presented to a pediatric emergency unit with acute epigastric pains. There was no complaint of fever, recent weakness, weight loss, abnormal stools containing occult or macroscopic blood. No aberrations were revealed during a physical examination. Laboratory testing was performed with no evidence of anemia or inflammation. The patient was admitted to the hospital to deepen the imaging diagnostics and find the cause of her condition. Ultrasonography (USG) revealed a mass within the stomach, so computed tomography (CT) was performed. It confirmed the presence of a tumor. Firstly, non-Hodgkin lymphoma (NHL) was suspected, yet after an incisional biopsy, it gave the suspicion of GIST. Unfortunately, the biopsy was complicated with bleeding.
The patient was referred to our clinic with an initial diagnosis of GIST. She underwent total surgical resection of the tumor in October 2016. Approximately 20% of the stomach was removed. The tumor was situated near the fundus and lesser curvature of the stomach. The excision was broadened due to another polyp near the cutting line. It was solid and gray on gross examination measuring more than 5 cm in the greatest diameter. The surgical margin was less than 0.1 mm. Microscopically the lesion consisted of a mixture of epithelioid tumor in nests and elongated cells arranged in fascicles resembling paraganglioma. The mitotic rate was 13/50 high-power fields (HPF) and neuro- and vascular invasion were found. The cells stained positive for antibodies against CD117, chloride channel protein anoctamin 1 (DOG1), CD99, smooth muscle actin (SMA), CD34, neuron specific enolase (NSE), vimentin, Ki 67 (in 5% of nuclei) and were negative for antibodies against desmin, chromogranin A, cytokeratin CK AE1/AE3, synaptophysin and S100. Based on morphology and immunoprofile, the pathology report confirmed the diagnosis of high-risk GIST (Miettinen’s prognostic group: 6a, pT3Nx). Pathology findings are shown on Figure 1.
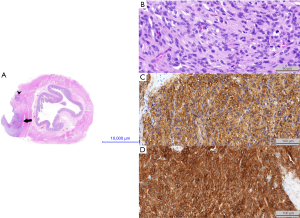
After the surgery and initial recovery, the patient was consulted in the outpatient clinic every 2 or 3 months with regular imaging check-ups—abdominal USG, CT or magnetic resonance imaging (MRI), and positron electron tomography (PET). In September 2017, another abdominal USG showed new and unclear soft tissue changes near the duodenum. This was not confirmed after another 2 months. PET examination in March 2018 showed uptake of 9 mm and standardized uptake value (SUV) =3.4 near the lesser curvature of the stomach that was not noted during the previous year’s PET. Although changes were not found in the exact anatomical location and the diameter of the new lesion was small, a biopsy under endoscopic ultrasound control was attempted. It revealed gastritis and no cancer pathology. USG performed in October 2018 depicted stably enlarged lymph nodes. The subsequent PET examination was performed in May 2019, showing the metabolic progression of the lesion found in March 2018 (SUV =6.9), also depicted in MRI imaging. CT performed in September 2019 demonstrated a 7 mm tumor situated submucosally in the posterior-lower wall of the pyloric sphincter, best seen in the arterial phase.
A retrospective genetic assessment of the specimen was performed in a certified laboratory. Targeted Sanger sequencing on KIT (exons 9, 11, 13, 17, reference sequence KIT LRG_307t1; LRG_307p1) and PDGFRA (exon 12, 14, 18, codon 842 in exon 18 and D842V mutation – PDGRFA LRG_309t1; p.LRG_309p1) was executed. The limit of detection was 20% of heterozygous cells with the mutation. The percentage of cancer cells in the tested material was >50%. The tissue fragment for DNA isolation containing cancer cells was extracted by macrodissection. The percentage of cancer cells exceeded the detection limit of the method used. KIT mutation in exons 13 and 17 was found; KIT exons 9 and 11 were impossible to be assessed. Based on the genetic aberrations found in the tumor, the biology of the tumor was classified as adult-type and such protocol was followed. In December 2019, imatinib therapy was introduced, at 400 mg daily (Institutional Bioethics Committee Agreement No. 996/19 on 30.10.2019). The disease stabilized for a year.
Another PET in January 2020 showed increased metabolic uptake of the lesion up to SUV =11.1, while the CT image was stable. Control PET in March 2020 showed metabolic uptake with SUV =6.0. CT performed in May 2020 revealed a lesion (8×12×8 mm3) on the border of the posterior and inferior wall of the pylorus with contrast uptake of 160 Hounsfield units (HU) in both arterial and venous phases. PET in October 2020 depicted 2 masses near the pyloric sphincter: the first 14 mm and SUV =8, the second 8 mm and SUV =2.5, finally correlating with the CT picture from December 2020, which is shown on Figure 2. Imatinib’s dose was escalated to 800 mg per day. The treatment with imatinib was complicated with facial edema, transient nausea and numbness of the upper and lower extremities that resolved within 3 weeks. Moreover, hypomagnesemia and mild anemia were noted, as well as alopecia and recurrent nasal hemorrhages that alleviated spontaneously.
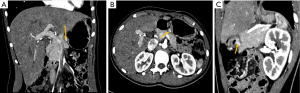
As the treatment intensification was ineffective and the tumor changes were localized and clearly seen in USG, the patient underwent subtotal gastric resection in April 2021 at the age of 15. The postoperative specimen consisted of two stomach fragments with the duodenum, one fragment of tumor and one lymph node. Grossly, the fragments of the stomach’s wall revealed 3 foci of solid infiltrative tumors (the biggest diameter of 2 cm) and some small polyps were found on the inner surface of the wall. The lymph node’s greatest diameter was 2.0 cm and tumor-free. The infiltrative tumor’s cells were stained for CD117 and were negative for antibodies against SMA, CD34, S100 and STAT6. The pathology report confirmed the recurrent disease in the pylorus and two satellite tumors near the body of the stomach. Repeated genetic screening in the same certified laboratory using Sanger sequencing of the same reference sequences revealed no KIT (exon 9, 11, 13, 17) or PDGFRA (exon 12, 14, 18, D842 mutation) aberrations in the recurrent disease. Germline control was not performed to date. Figure 3 shows the timeline of the patient’s medical history.
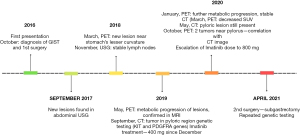
The patient is observed in the outpatient clinic following a high-risk GIST follow-up protocol (CT performed every 3 months in the first 3 years, every 6 months in the 4th and 5th years of follow-up, then yearly; PET executed every 6 months in the first 2 years, then annually; USG performed every 3 months).
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient and her parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Abdominal pain is one of the most frequent symptoms observed in pediatric patients. However, its differential diagnosis is difficult and tumors are a rare entity that may lead to such clinical presentation. Moreover, the pathophysiology of abdominal pains concerning underlying cancer may differ—from the neoplasm mass to hepatosplenic enlargement that compresses adjacent organs and provokes a feeling of fullness. A few types of tumors may be found in the abdomen of a pediatric patient, with NHLs the most frequently. Neuroblastoma, nephroblastoma, germinal tumors, or hepatoblastoma may be considered especially in the younger pediatric population, while in adolescents—hepatocarcinoma. Finally, pancreatoblastoma, gastrointestinal tract carcinomas, or GISTs belong to rare yet possible entities (10).
With NHL suspected at first, an incisional biopsy of the mass was performed to confirm this diagnosis and implement chemotherapy as soon as possible. Unfortunately, this preliminary diagnosis was rejected and the bleeding complicated the procedure. It is essential to remember that GIST is a very fragile tumor and any surgical manipulation should be done carefully with the whole tumor being removed with its capsule. Any bleedings or ruptures worsen the patient’s prognosis and may be the cause of the implementation of adjuvant chemotherapy with imatinib. It is highlighted that GI bleeding in GIST patients results in shorter relapse-free survival, especially in the high-risk group to which the patient was classified (11). Therefore, incisional biopsy should be fairly assessed as an error here. Reoperation in metastatic disease is legitimate as per the recommendations issued by Kikuchi et al. (12).
This case highlights imaging diagnostic difficulties as the disease’s progression was not seen adequately in each imaging technique, making the follow-up and the decisions about the next steps difficult. A watch-and-wait strategy was implemented to maximally lengthen the patient’s life and ensure its quality: any surgical or pharmacological treatment was started very cautiously.
Given that the patient’s first tumor harbored an adult-type mutation—KIT mutation in exons 13 and 17—adult treatment could be implemented. However, such mutations may not respond to imatinib (8). It was chosen in the first line of GIST targeted therapy following adult guidelines and doses as well as due to good accessibility. However, due to the observed lack of imatinib treatment efficacy, this case shows the necessity of running next-generation sequencing (NGS) of whole KIT, PDGFRA and SDH complex genes as early as possible (13,14). Facing a limited availability of NGS procedures as part of the diagnostic process can have consequences as in the following case. While there is no registered treatment for SDH-deficient GIST available, PDGFRA mutations allow for avapritinib administration. Moreover, other kinase inhibitors, such as sunitinib and regorafenib have been successfully used in pediatric population, providing disease stabilization, both in KIT-mutated and wild-type tumors (15). In the context of GIST dedifferentiation from adult-type into wild-type, previously not described, we can also speculate the need for extensive cancer genetic diagnostics, including SDH complex and TRK genes (due to the imatinib resistance), including fusions, RAS pathway genes (especially when RASopathies are suspected) (16,17), to predict more accurately the prognosis and apply better treatment, including larotrecinib or entrectinib (18-20).
Conclusions
Pediatric GISTs are usually characterized by distinct biology. As presented in this case report, they might be difficult in terms of imaging diagnostics and their genetic landscape may change in time, resulting in possible resistance to administered treatment. Therefore, there is a need to apply a non-standard diagnostic process and treatment. The case depicts the importance of complex genetic testing combined with histopathological diagnosis due to the treatment efficacy prediction. Moreover, it emphasizes the need for the establishment of unified diagnostics and treatment guidelines for pediatric gastrointestinal stromal tumors, including current possibilities of targeted treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1111/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1111/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1111/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient and her parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rutkowski P, Magnan H, Chou AJ, et al. Treatment of gastrointestinal stromal tumours in paediatric and young adult patients with sunitinib: a multicentre case series. BMC Cancer 2017;17:717. [Crossref] [PubMed]
- Al Atrash E, Abdullah MF, Pressey J, et al. GIST presenting as refractory iron-deficiency anaemia in paediatric patient. BMJ Case Rep 2022;15:e248365. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Rink L, Godwin AK. Clinical and molecular characteristics of gastrointestinal stromal tumors in the pediatric and young adult population. Curr Oncol Rep 2009;11:314-21. [Crossref] [PubMed]
- Benesch M, Wardelmann E, Ferrari A, et al. Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr Blood Cancer 2009;53:1171-9. [Crossref] [PubMed]
- Raitio A, Salim A, Mullassery D, et al. Current treatment and outcomes of pediatric gastrointestinal stromal tumors (GIST): a systematic review of published studies. Pediatr Surg Int 2021;37:1161-5. [Crossref] [PubMed]
- Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv267. [Crossref] [PubMed]
- Tornillo L, Terracciano LM. An update on molecular genetics of gastrointestinal stromal tumours. J Clin Pathol 2006;59:557-63. [Crossref] [PubMed]
- Willobee BA, Quiroz HJ, Sussman MS, et al. Current treatment strategies in pediatric gastrointestinal stromal cell tumor. Transl Gastroenterol Hepatol 2018;3:53. [Crossref] [PubMed]
- Golden CB, Feusner JH. Malignant abdominal masses in children: quick guide to evaluation and diagnosis. Pediatr Clin North Am 2002;49:1369-92. viii. [Crossref] [PubMed]
- Huang Y, Zhao R, Cui Y, et al. Effect of Gastrointestinal Bleeding on Gastrointestinal Stromal Tumor Patients: A Retrospective Cohort Study. Med Sci Monit 2018;24:363-9. [Crossref] [PubMed]
- Kikuchi H, Hiramatsu Y, Kamiya K, et al. Surgery for metastatic gastrointestinal stromal tumor: to whom and how to? Transl Gastroenterol Hepatol 2018;3:14. [Crossref] [PubMed]
- Yu J, Li Y, Li T, et al. Gene mutational analysis by NGS and its clinical significance in patients with myelodysplastic syndrome and acute myeloid leukemia. Exp Hematol Oncol 2020;9:2. [Crossref] [PubMed]
- Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Arch Pathol Lab Med 2017;141:751-8. [Crossref] [PubMed]
- Wang YM, Gu ML, Ji F. Succinate dehydrogenase-deficient gastrointestinal stromal tumors. World J Gastroenterol 2015;21:2303-14. [Crossref] [PubMed]
- Andrzejewska M, Czarny J, Derwich K. Latest Advances in the Management of Pediatric Gastrointestinal Stromal Tumors. Cancers (Basel) 2022;14:4989. [Crossref] [PubMed]
- Rinelli M, Agolini E, Milano GM, et al. Pediatric gastrointestinal stromal tumor: Report of two novel patients harboring germline variants in SDHB and SDHC genes. Cancer Genet 2020;241:61-5. [Crossref] [PubMed]
- Atiq MA, Davis JL, Hornick JL, et al. Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: a clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST). Mod Pathol 2021;34:95-103. [Crossref] [PubMed]
- Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med 2016;14:339. [Crossref] [PubMed]
- Demetri GD, Antonescu CR, Bjerkehagen B, et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: expert recommendations from the World Sarcoma Network. Ann Oncol 2020;31:1506-17. [Crossref] [PubMed]


