
Tumor mutation burden in gastro-entero-pancreatic-neuroendocrine neoplasms
Highlight box
Key findings
• This analysis showed that, despite very low incidence, there are GEP-NENs with high TMB. For precision medicine, testing for MSI and TMB is needed for this tumor type.
What is known and what is new?
• GEP-NENs are tumors with low TMB and MSS, which indicates the limited efficacy of ICIs in GEP-NENs, as found in previous clinical trials
• However, although very few, there are GEP-NENs with high TMB.
What is the implication, and what should change now?
• A test for MSI status and TMB is needed in this type of tumor for precision medicine.
Introduction
Neuroendocrine neoplasm (NEN) is a rare malignancy, with a 0.02% incidence (1). NENs can arise in almost every organ of the body. Although they share similar morphologic and immunophenotypic features, the primary anatomic site is an important classification criterion (2). The gastrointestinal tract (62–67%) and lung (22–27%) are the most common sites (1). Gastro-entero-pancreatic NENs (GEP-NENs) are classified according to the grading system based on proliferation assessed by mitotic rates and Ki-67 labeling; well-differentiated neuroendocrine tumor (NET) grades 1, 2, and 3 and poorly differentiated neuroendocrine carcinoma (NEC) (3). The prognosis of GEP-NENs is very poor, with a 5-year overall survival of 13%. More than 60% of patients with newly diagnosed GEP-NENs experience distant metastasis (4); however, the efficacy of systemic chemotherapy for GEP-NENs is limited (5).
The introduction of immune checkpoint inhibitors (ICIs) has changed the treatment strategies for various tumors. ICIs also showed promising results in some tumors with NEN features, such as small-cell lung cancer (6) and Merkel cell carcinoma (7). However, in GEP-NENs, the results were less promising. Because various biomarkers have been used to select patients who can benefit from ICIs, microsatellite instability (MSI) and tumor mutation burden (TMB) are novel biomarkers in various tumor types (8-10). However, these novel biomarkers have not been well studied in GEP-NENs.
Herein, we evaluated MSI status and TMB in patients with GEP-NENs. We present this article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1190/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board (IRB) of Samsung Medical Center approved this study (No. 2022-10-077-001). This study is a retrospective analysis. Therefore, the requirement for informed consent to this study was waived.
Patients selection
We selected patients diagnosed with GEP-NEN between 2013 and 2022 based on MSI status and TMB status, which were assessed using next-generation sequencing (NGS). The following patient clinicopathologic characteristics were analyzed: age, gender, pathologic diagnosis, site of metastasis, number of metastases, TMB, and MSI status. Pathologic diagnosis was re-reviewed by the pathologist according to the 2017 World Health Organization (WHO) classification of NENs (11).
Next-generation sequencing
Tumor samples were obtained at the time of initial diagnosis or progression, and. formalin-fixed paraffin-embedded (FFPE) material was used. The Qubit dsDNA HS Assay (Thermo Fisher Scientific, Waltham, MA, USA) on the Qubit 2.0 Fluorometer (Thermo Fisher Scientific) was used to quantify the 40 ng of DNA. The Covaris E220 Focused-ultrasonicator (Woburn, MA, USA) and the 8 microTUBE-50 Strip AFA Fiber V2 were used for shearing. The treatment time was optimized for FFPE material, and the treatment settings were as follows: peak incident power, 75 W; duty factor, 15%; cycles per burst, 500; treatment time, 360 s; temperature, 7 ℃; water level, 6. We used the TruSightTM Oncology 500 Kit (Illumina Inc., San Diego, CA, USA) for DNA library preparation and enrichment, following the manufacturer’s instructions. The post-enriched libraries were quantified, pooled, and sequenced on a NextSeq 500 (Illumina). The quality of the NextSeq 500 (Illumina) sequencing runs was assessed with the Illumina Sequencing Analysis Viewer (Illumina). TruSight Oncology 500 Local App Version 1.3.0.39 (Illumina) was used to analyze the sequencing data. The TruSightTM Oncology 500 is a comprehensive tumor profiling assay designed to identify various tumor biomarkers, including small variants, splice variants, and fusions. It also measures TMB and MSI, features that are potential key biomarkers for immunotherapy.
TMB was reported as mutations per megabase (Mb) sequenced. Although there is no consensus on the definition of the high TMB in NET, we use the cutoff of 10 mutations/Mb as a high TMB (12).
Statistical analysis
Descriptive statistics were used to summarize the characteristics of patients and tumors, MSI status, TMB, and treatment history. The data did not follow a normal distribution, and numerical variables were evaluated using the Mann-Whitney U test. All P values were two-sided, and statistical significance was set at P value <0.05. The statistical analyses were performed using IBM PASW version 25.0 software (SPSS Inc., Chicago, IL, USA).
Results
Clinical features
Between 2013 and 2022, 31 patients with GEP-NEN were evaluated for MSI status and TMB. We retrospectively review the medical records of those patients. The median age at the time of diagnosis was 61.7 [interquartile range (IQR), 53.5–68.9] years, and 18 (58.1%) patients were male (Table 1). According to the 2017 WHO classification of NENs, 1 (3.2%) patient was diagnosed with a grade 1 tumor, 15 (48.4%) patients with grade 2 tumors, and 3 (9.7%) patients with grade 3 tumors. NEC was diagnosed in 12 (38.7%) patients. The primary sites were as follows: 22 (71.0%) foregut-derived NET including stomach (4, 12.9%), pancreas (12, 38.7%), bile duct (1, 3.2%), gallbladder (4, 12.9%), liver (1, 3.2%); 2 (6.5%) midgut-derived NET [small bowel (2, 6.5%)]; and 7 (22.6%) hindgut-derived NET [rectum (7, 22.6%)]. The median number of metastases was 2.0 (IQR, 1.0–3.0; range, 0–4), and the most common metastatic sites were liver (23, 74.2%), lymph node (16, 51.6%), and lung (5, 16.1%).
Table 1
| Characteristics | Total number (n=31) |
|---|---|
| Male | 18 (58.1) |
| Age (years) | 61.7 (53.5–68.9) |
| Pathology | |
| Well-differentiated neuroendocrine tumor | 19 (61.3) |
| Grade 1 | 1 (3.2) |
| Grade 2 | 15 (48.4) |
| Grade 3 | 3 (9.7) |
| Neuroendocrine carcinoma | 12 (38.7) |
| Primary tumor site | |
| Stomach | 4 (12.9) |
| Small bowel | 2 (6.5) |
| Pancreas | 12 (38.7) |
| Bile duct | 1 (3.2) |
| Gallbladder | 4 (12.9) |
| Liver | 1 (3.2) |
| Rectum | 7 (22.6) |
| Number of metastases | 2.0 (1.0–3.0) |
| Site of metastasis | |
| Liver | 23 (74.2) |
| Lung | 5 (16.1) |
| Pancreas | 2 (6.5) |
| Pleura | 1 (3.2) |
| Peritoneum | 3 (9.7) |
| Adrenal gland | 2 (6.5) |
| Lymph node | 16 (51.6) |
| Bone | 2 (6.5) |
| Survived at the time of analysis | 18 (58.1) |
| TMB (mutations/Mb) | 4.7 (3.1–6.3) |
| MSI status | |
| MSS | 31 (100.0) |
Data are presented as median interquartile range or n (%). TMB, tumor mutation burden; MSI, microsatellite instability; MSS, microsatellite stability.
Tumor mutational burden and MSI
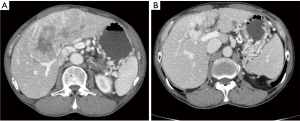
The median TMB score was 4.7 mutations/Mb (IQR, 3.1–6.3; range, 0.8–266.4; Figure 1) among 31 patients. Only 1 patient had a tumor with high TMB (266.4 mutations/Mb), and the tumor was classified as grade 3 NET with the pancreas as the primary tumor site. The patient received octreotide as the first-line, everolimus as the second-line, sunitinib as the third-line, and capecitabine plus temozolomide as the fourth-line treatment. The best tumor response was partial remission with capecitabine plus temozolomide (Figure 2). The duration of the response was 3 months. After progression, this patient was treated with 5-fluorouracil, irinotecan, and leucovorin combination therapy until the time of analysis.
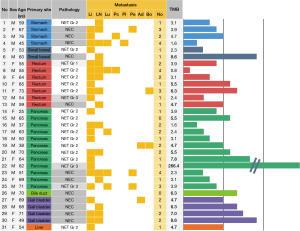
A statistically significant difference was not observed in the TMB score between well-differentiated NET (grade 1, 2, and 3) and poorly differentiated NEC (median: 3.9 vs. 4.7, P=0.232). In addition, the TMB score did not differ based on the primary tumor site (pancreatic NEN vs. other primary NEN; median: 3.9 vs. 4.7, P=0.646).
All 31 patients had tumors with microsatellite stability (MSS). The tumor with high TMB (266.4 mutations/Mb) was also an MSS tumor.
Discussion
In this analysis, 31 patients diagnosed with GEP-NEN had a median TMB of 4.7 mutations/Mb (IQR, 3.1–6.3; range, 0.8–266.4). The TMB value did not differ based on WHO grade or primary tumor site. Only 1 among 31 patients had a tumor with high TMB (266.4 mutations/Mb); this tumor was also MSS. Although the incidence of tumors with high TMB in GEP-NENs was very low, those with high TMB were observed, indicating the need for tests for MSI and TMB.
ICIs are a promising novel therapy in cancer treatment. Regarding GEP-NENs, several agents targeting programmed death-ligand 1 (PD-L1), programmed cell death protein 1 (PD-1), and cytotoxic T-lymphocyte-associate protein 4 (CTLA-4) have been investigated over the last few years. However, the outcomes reported in clinical trials are disappointing (Table 2). Thus, an important challenge is to discover biomarkers to select patients who are likely to benefit from ICIs. Currently, the well-known biomarkers of ICIs are PD-L1 expression (20), TMB (8), and MSI status (9,10). In the present study, we analyzed the status of MSI and TMB in GEP-NENs.
Table 2
| Drug name | Study phase | Total enrollment (n) | Inclusion | Diagnosis | Biomarker | ORR | Reference |
|---|---|---|---|---|---|---|---|
| Pembrolizumab | I | 41 | Advanced PD-L1 (+) pNET or carcinoids | pNET (n=16) | PD-L1 | PD-L1 (+) pNET: 6.3% (95% CI: 0.2–30.2%) | (13) |
| Pembrolizumab | II | 107 | W/D & M/D NET | Pancreas (n=40), SB (n=25), other GI (n=18) | PD-L1 | Overall: 3.7% (n=107); PD-L1 (+): 0% (95% CI: 0.0–19.5%) (n=17); PD-L1 (−): 4.8% (95% CI: 1.3–11.9%) (n=83) | (14) |
| Pembrolizumab | II | 29 | G3 NET | Pancreas (n=10), non-pancreatic GI (n=14) | PD-L1 | 1 (3.4%) | (15) |
| Toripalimab | I | 40 | NEN | Pancreas (n=9), GI (n=23) | PD-L1, TMB, MSI | 20% (n=14); better with PD-L1 (+), TMB-H, MSI-H | (16) |
| Nivolumab and Ipilimumab | II | 32 | Nonpancreatic NEN | GEP (n=15) | – | 25% (95% CI: 13–42%) | (17) |
| Nivolumab and Ipilimumab | II | 29 | Advanced NET | GEP (n=10) | – | 24% (n=7/29) | (18) |
| Spartalizumab | II | 32 | W/D NET or GEP-NEC | W/D NET (n=95), GI (n=32), pancreatic (n=33), GEP-NEC (n=21) | PD-L1 | NET: 7.4% (95% CI: 3.0–14.6); NEC: 4.8% (95% CI: 0.1–23.8) | (19) |
NET, neuroendocrine tumor; PD-L1, programmed death-ligand 1; pNET, pancreatic neuroendocrine tumor; CI, confidence interval; W/D, well-differentiated; M/D, moderately-differentiated; SB, small bowel; GI, gastrointestinal; NEN, neuroendocrine neoplasm; TMB, tumor mutation burden; MSI, microsatellite instability; TMB-H, high TMB; MSI-H, high MSI; GEP, gastro-entero-pancreatic; NEC, neuroendocrine carcinoma.
Because NENs are rare tumors, few studies have evaluated TMB in NENs, especially GEP-NENs. Previous studies analyzing TMB in NENs with variable inclusion criteria are summarized in Table 3. Most studies also reported low TMB and low rate of MSI-high in NENs, which is consistent with our analysis (21,22,27).
Table 3
| Diagnosis | Total enrollment (n) | TMB (mutations/Mb) | MSI-H | PD-L1 (+) | Reference | ||
|---|---|---|---|---|---|---|---|
| Median | IQR | Range | |||||
| GEP-NET | 31 | 4.7 | 3.1–6.3 | 0.8–266.4 | 0 | – | Our data |
| Pancreatic NET | 75 | Average: 5.8 | – | – | 0 | 2/70 (2.9%) | (21) |
| GI NEC | 29 | 5.68 | – | 0.57–11.75 | 0 | 9/31 (29.0%) | (22) |
| Metastatic and locally advanced NEN | 85 | 5.45 | 3.84–8.85 | – | – | – | (23) |
| Pulmonary NET | 48 | 0.31 | 0.22–0.67 | – | – | – | (24) |
| NET | 164 | 5.2 | 2.6–10.4 | – | – | – | (25) |
| High-grade GEP-NEN | 135 | Average: 9.5 | – | – | 4% | 6% | (26) |
| Low-grade GEP-NEN | 335 | Average: 5.1 | – | – | 0% | 1% | (26) |
TMB, tumor mutation burden; NET, neuroendocrine tumor; IQR, interquartile range; MSI-H, high microsatellite instability; PD-L1, programmed death-ligand 1; GEP, gastro-entero-pancreatic; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm.
However, in our study, there are no statistically significant differences between TMB of well-differentiated NET and poorly-differentiated NEC. One study only analyzed NEC also reported low median TMB (5.68 mutations/Mb) (22). However, another study with a large number of patients reported higher TMB in high-grade GEP-NEN than in low-grade GEP-NEN (26). This study also reported 4% of MSI-high tumors in high-grade GEP-NEN, which is a relatively high rate comparing other studies. Those studies all had different inclusion criteria, therefore, further study would be needed.
There was only one TMB-high tumor in this study. This tumor was grade 3 NET of the pancreas and the liver biopsy was done. This tumor was MSS and TMB was 266.4 mutations/Mb. Ki-67 level was 55%.
Previously, a single case of grade 3 NET of the pancreas with temozolomide-induced high TMB was reported (28). In the present analysis, 2 patients underwent the NGS test after temozolomide-based therapy and this patient with TMB-high tumor is one of the two. However, the other patient diagnosed with grade 2 NET of the pancreas did not show high TMB (7.8 mutations/Mb). Although there is the possibility of treatment options for ICIs after certain treatments, further studies are needed.
There are some limitations to our study. First, due to the rarity of this disease, this study was based on a small sample size. Second, this was a retrospective study that only included the patients who had NGS. Therefore, there are possibilities that patients with the more advanced stage were included. Further studies with large and various samples would be needed in the future.
Conclusions
In conclusion, GEP-NENs are tumors with low TMB and MSS, which indicates the limited efficacy of ICIs in GEP-NENs, as found in previous clinical trials (Table 2). However, although very few, there are GEP-NENs with high TMB. A test for MSI status and TMB is needed in this type of tumor for precision medicine.
Acknowledgments
This study was posted in the ASCO-GI 2023.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1190/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1190/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1190/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1190/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board (IRB) of Samsung Medical Center approved this study (No. 2022-10-077-001). This study is a retrospective analysis. Therefore, the requirement for informed consent to this study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology 2004;80:3-7. [Crossref] [PubMed]
- La Rosa S, Uccella S. Classification of neuroendocrine neoplasms: lights and shadows. Rev Endocr Metab Disord 2021;22:527-38. [Crossref] [PubMed]
- Rindi G, Mete O, Uccella S, et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol 2022;33:115-54. [Crossref] [PubMed]
- Dasari A, Mehta K, Byers LA, et al. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018;124:807-15. [Crossref] [PubMed]
- Paulson AS, Bergsland EK. Systemic therapy for advanced carcinoid tumors: where do we go from here? J Natl Compr Canc Netw 2012;10:785-93. [Crossref] [PubMed]
- Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol 2019;12:47. [Crossref] [PubMed]
- Femia D, Prinzi N, Anichini A, et al. Treatment of Advanced Merkel Cell Carcinoma: Current Therapeutic Options and Novel Immunotherapy Approaches. Target Oncol 2018;13:567-82. [Crossref] [PubMed]
- Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther 2017;16:2598-608. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. [Crossref] [PubMed]
- Inzani F, Petrone G, Rindi G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol Metab Clin North Am 2018;47:463-70. [Crossref] [PubMed]
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353-65. [Crossref] [PubMed]
- Mehnert JM, Bergsland E, O'Neil BH, et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020;126:3021-30. [Crossref] [PubMed]
- Strosberg J, Mizuno N, Doi T, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res 2020;26:2124-30. [Crossref] [PubMed]
- Vijayvergia N, Dasari A, Deng M, et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer 2020;122:1309-14. [Crossref] [PubMed]
- Lu M, Zhang P, Zhang Y, et al. Efficacy, Safety, and Biomarkers of Toripalimab in Patients with Recurrent or Metastatic Neuroendocrine Neoplasms: A Multiple-Center Phase Ib Trial. Clin Cancer Res 2020;26:2337-45. [Crossref] [PubMed]
- Patel SP, Othus M, Chae YK, et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res 2020;26:2290-6. [Crossref] [PubMed]
- Klein O, Kee D, Markman B, et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin Cancer Res 2020;26:4454-9. [Crossref] [PubMed]
- Yao JC, Strosberg J, Fazio N, et al. Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocrine-Related Cancer 2021;28:161-72. [Crossref] [PubMed]
- Khunger M, Hernandez AV, Pasupuleti V, et al. Programmed Cell Death 1 (PD-1) Ligand (PD-L1) Expression in Solid Tumors As a Predictive Biomarker of Benefit From PD-1/PD-L1 Axis Inhibitors: A Systematic Review and Meta-Analysis. JCO Precis Oncol 2017;1:1-15. [Crossref] [PubMed]
- Salem ME, Puccini A, Grothey A, et al. Landscape of Tumor Mutation Load, Mismatch Repair Deficiency, and PD-L1 Expression in a Large Patient Cohort of Gastrointestinal Cancers. Mol Cancer Res 2018;16:805-12. [Crossref] [PubMed]
- Xing J, Ying H, Li J, et al. Immune Checkpoint Markers in Neuroendocrine Carcinoma of the Digestive System. Front Oncol 2020;10:132. [Crossref] [PubMed]
- van Riet J, van de Werken HJG, Cuppen E, et al. The genomic landscape of 85 advanced neuroendocrine neoplasms reveals subtype-heterogeneity and potential therapeutic targets. Nat Commun 2021;12:4612. [Crossref] [PubMed]
- Sullivan I, Kossai M, Le Teuff G, et al. MA11.02 Mutational Burden in Pulmonary Neuroendocrine Tumors (puNETs). J Thorac Oncol 2017;12:S404. [Crossref]
- Shao C, Li G, Huang L, et al. Prevalence of High Tumor Mutational Burden and Association With Survival in Patients With Less Common Solid Tumors. JAMA Netw Open 2020;3:e2025109. [Crossref] [PubMed]
- Puccini A, Poorman K, Salem ME, et al. Comprehensive Genomic Profiling of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Clin Cancer Res 2020;26:5943-51. [Crossref] [PubMed]
- Arnason T, Sapp HL, Rayson D, et al. Loss of expression of DNA mismatch repair proteins is rare in pancreatic and small intestinal neuroendocrine tumors. Arch Pathol Lab Med 2011;135:1539-44. [Crossref] [PubMed]
- Cao Y, Ma Y, Yu J, et al. Favorable response to immunotherapy in a pancreatic neuroendocrine tumor with temozolomide-induced high tumor mutational burden. Cancer Commun (Lond) 2020;40:746-51. [Crossref] [PubMed]


