Nab-paclitaxel as alternative treatment regimen in advanced cholangiocellular carcinoma
Introduction
The incidence of intrahepatic cholangiocarcinoma (ICD-10: C22.1) was estimated as 0.9–1.3 and 0.4–0.7/100,000 for males and females, respectively (1). In concordance with previous observations in the US (2) and Western European Countries (3,4), the incidence of intrahepatic biliary cancer increased within the last few years by approximately 5-fold (5).
Chemotherapeutic treatment options for advanced and metastatic biliary tract cancer still remain limited due to the heterogeneity of the disease and the poor understanding of profound molecular mechanisms (6). The standard treatment regimen for patients suffering from advanced intrahepatic cholangiocarcinoma represents a combination of gemcitabine and cisplatin (7). Compared to gemcitabine monotherapy, combined treatment with cisplatin resulted in a significant increase in overall survival (OS) (11.7 vs. 8.1 months, P<0.001) as well as progression free survival (PFS) (8.0 vs. 5 months, P<0.001) in a recently published randomized phase III trial (7).
A retrospective analysis of Walter et al. suggested a defined subpopulation with advanced biliary tract cancer that is suitable for second line treatment (8). In this particular analysis, 25% of younger and physically fit patients received further chemotherapy, most frequently combination regimens. Disease control was observed in 43%, and PFS and OS was 2.8 and 7.5 months, respectively (8). There are only few additional studies providing evidence that second line treatment in advanced biliary tract cancer is efficient (9).
Albumin-bound paclitaxel particles [nab-paclitaxel (Abraxane), Celgene] revealed antitumor activity as a single agent and synergistic activity in combination with gemcitabine in metastatic pancreatic cancer (10). In addition to pancreatic cancer, nab-paclitaxel was approved by the FDA and EMEA in the treatment of advanced breast cancer after failure of previous chemotherapy. Recently, the FDA also approved nab-paclitaxel combination therapy with carboplatin in non-small cell lung cancer. In preclinical studies, albumin-bound paclitaxel (nab-paclitaxel) showed antitumor activity as a single agent and had synergistic activity in combination with gemcitabine in murine models of pancreatic cancer (11,12). Especially in advanced pancreatic cancer, a disease in which many clinical phase III trials failed to show significant benefit of new agents, the introduction of nab-paclitaxel in combination with gemcitabine has become a novel standard treatment (11). Nab-paclitaxel was thereby found to enhance intra-tumoral concentrations of gemcitabine, thus leading to tumor shrinkage (12).
We retrospectively analyzed a single center registry of patients with advanced cholangiocellular carcinoma who received Nab-paclitaxel as second or third line treatment. We identified 12 patients who received nab-paclitaxel in combination either with gemcitabine or with fluoropyrimidines. All patients were analyzed retrospectively for treatment efficacy in terms of disease control rate (DCR), PFS and OS.
Methods
Study design
The study was conducted in accordance with the International Conference on Harmonization E6 requirements for Good Clinical Practice and with the ethical principles outlined in the Declaration of Helsinki (13).
This retrospective study was designed by the Comprehensive Cancer Study Group of the Medical University of Vienna.
Patients
Eligible patients had histologically confirmed diagnosis of intrahepatic cholangiocellular carcinoma (ICD-10: C22.1) that were non-resectable and metastatic. Measurable disease according to RECIST 1.1 criteria was required. Patients who received nab-paclitaxel after platinum-based treatment failure were eligible for analysis. Other eligibility criteria at baseline included an ECOG performance status of 0–2; a measured or calculated creatinine clearance of >60 mL·min−1; adequate bone marrow function indicated by a leukocyte count 3,000 mcL−1, absolute neutrophil count 1,000 mcL−1 and platelet count 100,000 mcL−1; adequate hepatic function with a total bilirubin up to 1.5× the institutional upper limit of normal.
Treatment plan and toxicity assessment
Patients were treated with a nab-paclitaxel based combination therapy after failure of a standard treatment regimen. Gemcitabine (1,000 mg per square meter of body-surface area) or fluoropyrimidine were applied weekly for 3 weeks in a 4-week cycle (d 1, 8, 15; q =28 d) in combination with nab-paclitaxel (125 mg per square meter of body-surface area).
Toxicities were graded by the National Cancer Institute Common Terminology Criteria for adverse events version 4.0. Treatment with both drugs was held for grade 3 or 4 toxicity, and subsequently reduced in dose by 25%.
Disease assessment
Objective response was assessed every 8–12 weeks or after 3 cycles of drug application using the Response Evaluation Criteria in Solid Tumors classification 1.1. PFS was calculated from the date of registration to the date of first observation of progressive disease (PD), death due to any cause or symptomatic deterioration. Patients known to be alive and progression free were censored at last date of contact.
Statistical considerations
Study population was characterized retrospectively in a single center analysis. Patients who were treated at the Department of Internal Medicine I, who suffer from biliary tract cancer, were evaluated by descriptive statistics. OS and PFS were analyzed with the use of SAS Enterprise Guide 6.1 and described as Kaplan-Meier curves.
Results
Patient characteristics
From 09/2012 to 04/2015, a total of 12 patients, comprising 6 (50%) women and 6 (50%) men, were identified. Clinical characteristics are summarized in Table 1. The mean age of these patients was 54±12 years. All patients had an ECOG 0–1 performance score, and all patients had metastatic lesions from which most were located in the liver (83%).
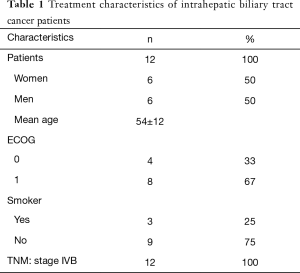
Full table
First line treatment
Nine of 12 patients were treated with gemcitabine/cisplatin as first line treatment. One patient received gemcitabine in combination with oxaliplatin and 1 patient received FOLFOX (Table 2). From all 12 patients, five received nab-paclitaxel as 2nd (42%), and 7 patients as 3rd (58%) line treatment. In 6 (50%) patients, nab-paclitaxel was combined with gemcitabine, in 6 (50%) patients, nab-paclitaxel was combined with a fluoropyrimidine based chemotherapeutic regimen (Table 3).

Full table

Full table
Toxicities
Among the 12 patients, fever was documented in 3 patients (25%; Table 4). Two patients (17%) developed edema and neuropathy, respectively. Other toxicities described during the observation time were anemia, headache, thrombosis, ototoxicity and vertigo (all n=1; 8%). All side effects were rated mild (grade 1 or 2; Table 4).

Full table
Clinical efficacy
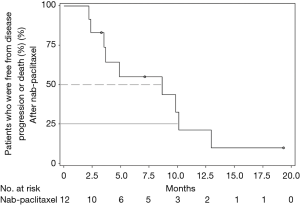
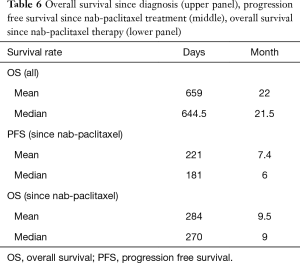
All 12 patients were eligible for response, with 1 patient achieving complete remission (CR) and 2 patients achieving partial remission (PR) resulting in an overall response rate (ORR) of 25%. Stable disease (SD) was observed in 7 patients (58%) and PD in 2 (17%). Thus, the DCR was 83% (Table 5). The median time of survival after diagnosis of advanced disease was 21.5 months (Figure 1), whereby 3 patients were alive at the date of censoring (04/01/2015). Median OS after initiation of nab-paclitaxel treatment was 9 months (2.1–28.4 months; Figure 2). Median PFS after initiation of nab-paclitaxel application was 6 months (2.1–19.5 months; Figure 3, Table 6).
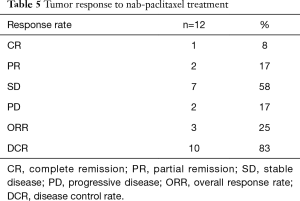
Full table
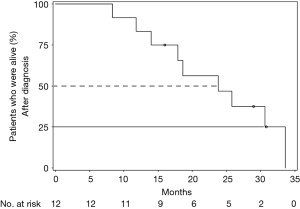
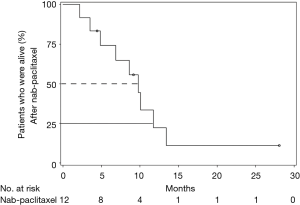
Full table
Discussion
Chemotherapy-treated biliary tract cancer patients showed enhanced OS and increase in quality of life when compared with patients receiving best supportive care alone as first-line palliative treatment (14).
Recent advances in the treatment of malignant tumors implicated the introduction of biological therapies including monoclonal antibodies and tyrosine kinase inhibitors (15). However, in locally advanced as well as metastatic biliary tract cancer, such novel therapeutic approaches failed to provide clinical benefit (16,17). In adenocarcinoma of the biliary tract, overexpression of the epidermal growth factor receptor (EGFR) or the vascular endothelial growth factor receptor (VEGFR) have been found in approximately 27% and 59%, respectively (16). Clinical phase II trials of sorafenib (400 mg BID) or erlotinib (100 mg daily) in patients with advanced biliary cancer, however, failed to show a meaningful benefit (18). The addition of cetuximab to standard chemotherapy in the BINGO trial also failed to reveal benefit when compared to chemotherapy only (19).
As gemcitabine is part of the first line therapy to treat patients suffering from biliary tract cancer and nab-paclitaxel was described to support gemcitabine effects on OS in pancreatic cancer, we aimed to evaluate the effects of nab-paclitaxel in the treatment of cholangiocellular carcinoma. Following failure of first line treatment no second line therapy has been established in clinical routine. Recent systematic reviews of second line chemotherapies in cholangiocarcinomas suggested a cohort of patients, which may benefit from further chemotherapeutic treatment with an increase in OS (20). In phase II second-line chemotherapy trials, patients were treated with various drugs including docetaxel or erlotinib (17), 5-FU in combination with doxorubicin and mitomycin C (21), irinotecan (22) or targeted therapies like everolimus (23) or imatinib (24). Median OS for second line treatment in a systematic review including 20 studies was 7.2 months (95% CI, 6.2–8.2), while median PFS was 3.2 months (20). While different mono- as well as combination chemotherapies and tyrosine kinase inhibitors were tested for their biological activity in this setting, we provided first evidence that Nab-paclitaxel in combination with chemotherapy may be beneficial after failure of platinum-based treatment. Results indicate a median OS, following initiation of nab-paclitaxel salvage treatment, of 9 months and a PFS of 6 months. The therapeutic potential of nab-paclitaxel in combination with gemcitabine or fluoropyrimidine as second or third line treatment was underlined by the fact that 83% of our patients showed disease control. Notably, the subset of patients described here had a median OS since diagnosis of non-resectable disease of 21.5 months. The experimental nab-paclitaxel combination therapy was associated with only few and mild side effects such fever (25%) and neuropathy (17%).
The study has several limitations. It was a non-randomized and retrospective analysis of a single institutional registry. The small number of patients identified, are lacking an adequate control group and all patients had a good performance status (ECOG 0–1). However, to the best of our knowledge this is the first report describing a clinical benefit of nab-paclitaxel in metastatic cholangiocellular carcinoma. Therefore, a first presentation of this substance as a possible treatment regimen is significantly relevant. While NCT 02392637 is currently investigating the therapeutic potential of gemcitabine plus nab-paclitaxel in first line treatment, additional randomized trials are needed.
Acknowledgements
This work was supported by the Comprehensive Cancer Center Vienna at the Medical University of Vienna.
Footnote
Conflicts of Interest: W Scheithauer—Consultant & advisory role, honoraria and research funding, Celgene Corporation; G Kornek and G Prager—honoraria as invited speakers, Celgene Corporation.
Ethical Statement: The study was approved by institutional ethics board (NO. EK 274/2011) and all of the patients have signed an ICF agreeing with academic evaluation of this retrospective analysis.
References
- Eckel F, Brunner T, Jelic S, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22 Suppl 6:vi40-4. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- Lepage C, Cottet V, Chauvenet M, et al. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol 2011;54:306-10. [Crossref] [PubMed]
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [Crossref] [PubMed]
- Pinter M, Hucke F, Zielonke N, et al. Incidence and mortality trends for biliary tract cancers in Austria. Liver Int 2014;34:1102-8. [Crossref] [PubMed]
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657-69. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013;49:329-35. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs 2011;29:1488-93. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [Crossref] [PubMed]
- Frese KK, Neesse A, Cook N, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2012;2:260-9. [Crossref] [PubMed]
- Good clinical practice research guidelines reviewed, emphasis given to responsibilities of investigators: second article in a series. J Oncol Pract 2008;4:233-5. [Crossref] [PubMed]
- Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. [Crossref] [PubMed]
- Bizama C, García P, Espinoza JA, et al. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev 2015;41:222-34. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]
- Chiorean EG, Ramasubbaiah R, Yu M, et al. Phase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06-101. Oncologist 2012;17:13. [Crossref] [PubMed]
- El-Khoueiry AB, Rankin C, Siegel AB, et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer 2014;110:882-7. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref] [PubMed]
- Lim KH, Han SW, Oh DY, et al. Outcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancer. Oncology 2012;83:57-66. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. A pilot study of salvage irinotecan monotherapy for advanced biliary tract cancer. Anticancer Res 2013;33:2619-22. [PubMed]
- Buzzoni R, Pusceddu S, Platania M, et al. Efficacy and safety of RAD001 I advanced biliary tract cancer patients progressing after first-line chemotherapy: a phase II study. J Clin Oncol 2010;28;abstr e14500.
- Roth A, Schleyer E, Schoppmeyer K, et al. Imatinib mesylate for palliative second-line treatment of advanced biliary tract cancer: a bicentric phase II study. Onkologie 2011;34:469-70. [Crossref] [PubMed]


