
Effects of polymer terminal group inside micelle core on paclitaxel loading promoting and burst release suppressing
Highlight box
Key findings
• Compared with the HOOC-PBMA-PNAM-Phen, the micelles with Phen group on the hydrophobic block (HOOC-PNAM-PBMA-Phen) enhanced drug loading and prolonged drug release, but larger particle size.
What is known and what is new?
• To control the release of drugs according to the actual clinical needs, many methods, such as micelles and emulsions, have been tried out but they all come up with limited effects, especially on the control of the explosive release of drugs.
• The hydrophobic end groups on the hydrophobic blocks seem much better for the hydrophobic drug such as PTX loading, which might suppress the undesired burst drug release in pharmacokinetics.
What is the implication, and what should change now?
• The hydrophobic end group Phen on the core-forming blocks can promote hydrophobic drug loading and suppress burst of drug release.
Introduction
Paclitaxel (PTX) is widely used in the treatment of esophageal and gastric cancer (1,2). PTX is insoluble in water, limiting its anti-tumor effect. Nanotechnology is expected to solve this problem (3,4). Block copolymers have been synthesized by various methods over the past few decades for building micelles for hydrophobic drug delivery (5,6). Amphiphilic block copolymers containing both hydrophilic and hydrophobic segments are known for their ability to self-assemble into nano-organized morphologies (e.g., micelles, nanobubbles, or nanorods) when being dissolved in a solvent that selectively dissolves one of the two segments (7-10). The aggregation of these copolymers, which leads to the formation of micelles, is driven by adverse interactions between selective solvents and insoluble blocks. The blocks tend to aggregate and minimize their contact with the solvent. This process produces micellar systems comprising core–shell structures, with a corona consisting of the soluble blocks. In aqueous media, these amphiphilic block copolymers can form spherical micelles with a core/shell structure (11). Typically, the core is formed by hydrophobic block, and the shell is formed by hydrophilic block (12).
These polymeric micelles have some unique features making them effective carrier systems (12-14). Firstly, the polymeric micelles may be water-soluble if the solubility of the number of monomers in the shell-forming block is greater than the number of monomers in the core-forming block. This hydrophilicity can stabilize polymer micelles core and further load the hydrophobic chemotherapy drug in water systems. It also effectively prevents protein adsorption and cell adhesion, thus helping micelles avoid recognition as they circulate in the blood (15). Secondly, the hydrophobic blocks serve as nano-reservoirs for the loading and release of hydrophobic drug molecules such as PTX that are conjugated or complexed with the polymeric backbone, or physically encapsulated in the core by similar-to-similar interaction (16). Thirdly, polymeric micelles are often serum-stable than other particles in vivo. Nanoscale delivery systems have relatively long circulation time and are amenable to extravasation at permeable tumor sites (17-19). Various organs of the human body are suitable for nano-carriers of different sizes (12).
Traditionally, for the hydrophobic drug delivery system based on above-mentioned micelles, scientists have generally focused on ways to improve micellar properties such as working on the temperature, pH and re-dox response for improving the drug accumulation at tumor site. Actually, the drug-loading efficiency and the release kinetics depend strongly on the amphiphilic block copolymers, which are key factors on the chemotherapy. This depends on various factors, such as their molecular weight and composition, the chemical structure of the diblock copolymer, the molecular volume of solubilization, the interfacial tension with regard to water, and the length of the core- and shell-forming blocks in the copolymer (20,21).
Typically, the block copolymers are prepared by the well-known reversible addition–fragmentation chain-transfer polymerization (RAFT) resulting in hydrophilic end group on the hydrophobic blocks while hydrophobic end group on the hydrophilic blocks. The stability of the micellar structure is a prerequisite for control over the rate of drug release. Drug release is dependent on the rate of drug diffusion through the micelle, or the rate of break-up of the micelle when it physically encapsulates the drug. The physical states of the micelle core and the encapsulated drug also play an important role (22). Jiaying et al. have reported that the drug-loading efficiency of nanoparticles increases dramatically when the carboxylic acid content of the block copolymer is increased (23). In contrast, the drug release rate from nanoparticles comprising copolymers with high carboxylic acid contents is much lower. These results may be associated with extensive interactions between the carboxylic groups of the copolymers and the drug (24,25). Moreover, functional groups contained in the hydrophobic or hydrophilic block of the copolymer have important effects on drug-loading efficiency and release kinetics (26,27).
As evaluated by the Flory-Huggins interaction parameter, the similar-to-similar interaction was very important in the drug-loading efficiency and release kinetics of copolymer (28). Moreover, as most of the chemotherapy drugs are insolvable in water, it is reasonable that the hydrophobic end groups on the hydrophobic blocks seem much better for the hydrophobic drug, as in the case of PTX loading which might suppress the undesired burst drug release in pharmacokinetics.
In the present study, the chemical structure and composition of the diblock copolymer, especially the effects of end group on the core-forming blocks on the miracle drug loading and release were systemically investigated. To do so, PBMA-PNAM diblock copolymers was synthesized by RAFT. The end group effects on PTX loading capacity and release behavior were systemically compared. We present this article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-206/rc).
Methods
Firstly, PBMA-b-PNAM was prepared. Secondly, the micelles were self-assembled in the water. Thirdly, the size distribution was determined by dynamic light scattering, and the micelle morphology was determined by transmission electron microscopy (TEM). Fourthly, the PTX loading and releasing profile were verified by reverse-phase high-performance liquid chromatography (r-HPLC). Lastly, the thermal stability and thermal transition behavior of the polymer were studied by differential scanning calorimeter (DSC).
Materials
The chemical reagents and materials were consistent with the previous published papers (28-30). The Milli-Q water was purified by Milli-Q A10 water purification system (Millipore, Billerica, MA, USA). A 4-cyanopentanoic acid dithiobenzene (CPADB) RAFT agent was prepared using previously described methods with some modifications.
Preparation of PBMA-b-PNAM
Diblock copolymer synthesis by RAFT
The PBMA-b-PNAM nanoparticles were synthesized as follows [PBMA, poly(n-butyl methacrylate); PNAM, poly(N-acryloylmorpholine)]. As shown in Figure 1A, hydrophobic PBMA homopolymers with approximately 28 or 62 monomer units were prepared using CPADB as a chain transfer agent (CTA). BMA (1.0 M for BMA60 or 2.0 M for BMA62), CPADB (25 mM), and V501 (5 mM) were dissolved in 50 mL of benzene. The solution was depressurized and degassed during the thawing cycle of three refrigeration pumps. The polymerization was carried out for 7 h in a pre-heated water (70 ℃). After polymerization, the polymer was fully precipitated in methanol/water mixture for 3 times (30% water by volume), then thoroughly dried under vacuum. The obtained pink PBMA60 and PBMA62 polymers, which were to be used as macro-CTAs, were stored at low temperature. PBMA-b-PNAM block copolymers with various hydrophobic and hydrophilic block lengths, in which the hydrophobic and hydrophilic monomer unit numbers were written as BMAN and NAMN, were synthesized by conventional RAFT polymerization, as described in the previous literature (8,24,25,29-31).
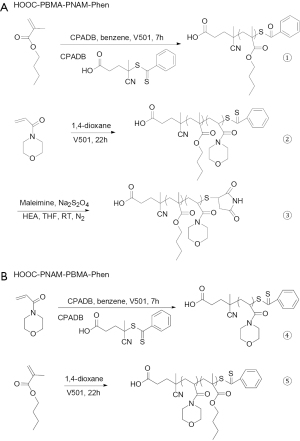
PBMA-b-PNAM diblock copolymers were synthesized using PBMA60 or PBMA62 as the macro-CTA, as shown in Figure 1B. The weight of the monomer was calculated in proportion. NAM (1.0 and 2.0 M), PBAM (10 mM), and V501 (2.0 mM) were dissolved in 10 mL of 1,4-dioxane. The solution was depressurized and degassed during the thawing cycle of three refrigeration pumps. The polymerization was carried out over approximately 22 h in a pre-heated water (70 ℃). After polymerization, the solution was precipitated in ether for 3 times. Then, dry under vacuum to recover the solid powder.
Aminolysis and conversion of polymer termini
Figure 1A shows the conversion of a dithiobenzoate end group to a hydrophilic maleimide group. Briefly, a propositional amount of the PBMA-b-PNAM diblock copolymer (0.015 mmol), 2 mol equivalents of Na2S2O4, and 40 mol equivalents of maleimide (versus the terminal groups) were dissolved in 5 mL of tetrahydrofuran (THF). THF was reduced by N2 for 1 h. 2-ethanolamine in 1mL of pre-deoxidized THF was slowly dripped into the polymer solution as N2 bubbles through. Let the mixture react at 24 ℃ for 20 h. When the reaction was complete, the solution was dialyzed against Milli-Q water (Millipore, CA, USA) using a dialysis membrane (MWCO =1,000 D) until the unreacted chemicals and THF had been completely removed. Finally, the white polymer product containing maleimide end groups was recovered by freeze-drying (32).
Characterization of PBMA-b-PNAM
The number-average molecular weight of the PBMA-b-PNAM diblock copolymer was detected by the proton nuclear magnetic resonance (1H NMR) spectra (400 MHz) as previously described in the literature (32). Tetramethyl silane was used as internal standard, and deuterated chloroform (CDCl3) was used as solvent.
Fabrication and characterization of the micelles
Fabrication of the micelles
The micelles were prepared by the method described in the literature (8,24,25,29-31). The resulting solution had a calculated micelle concentration of 2.0 mg/mL and was stored until required for further characterization.
Size distribution determined by dynamic light scattering
The hydrodynamic diameter and size distribution were detected by dynamic light scattering (DLS) using ZetaSizer Nano-ZS (Malvern Instruments, Malvern, UK). The micelle concentration of the sample was 2.0 mg/mL.
Micelle morphology determined by TEM
We prepared stained specimens for typical transmission electron microscopy experiments using a Hitachi H-7000 electron microscope as previously described in the literature (32).
Calibration of PTX by r-HPLC
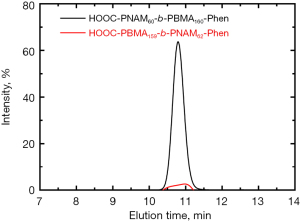
We verified the PTX concentration by HPLC using LiChrosorb RP-18 (Agilent Technologies Inc, USA). The mobile phase was water (A)/acetonitrile (B) (v/v =60:50). The maximal absorption for PTX was at 11 min. The concentration of PTX was about 0.00243, 0.00814, 0.0252, 0.0744, and 0.221 mg/mL for the calibration.
PTX loading
Preparation of the PTX–micelle complex
The PTX was dissolved in ethanol with concentrations around 5.0 mg/mL. Then 0.3 mL of the PTX solution was added to 3 mL of the micelle solution. To completely remove the ethanol, the resulting mixed solution was then dialyzed with Milli-Q water for 3 h at 24 ℃ using a dialysis membrane (MWCO =1,000 D). The loading content was calculated by the following equation:
Determination of the PTX loading level
The size and stability of the PTX-micelle complex was then obtained by DLS. The PTX loading level was evaluated by HPLC by mixing approximately 0.5 mL of AN with 0.5 mL of the PTX-micelle solution. Then HPLC was used to determine the PTX content with a UV detector at 227 nm and calculated by the above-mentioned calibration.
PTX release
A dialysis bag containing approximately 6.0 mL of the PTX-micelle solution was placed in a beaker with 1,000 mL of Mill-Q water at pH 7.4. The C0 of PTX in the micelles was approximately 0.15 mg/mL. In PBS solution, the PTX-micellar mixture was continued dialysis. The HPLC was used to analyze the Ct of PTX at fixed time.
Differential scanning calorimeter
The thermal stability and thermal transition behavior of the polymer were studied by DSC. DSC carried out nitrogen atmosphere on Netzsch 204F1 system. Indium standard was used for temperature and energy calibration. Each sample was heated from 25 to 200 ℃, maintained at this temperature for 3 minutes to eliminate its thermal history, cooled to room temperature, and finally reheated from 25 to 200 ℃. The heating or cooling rate was 10 ℃/min.
Statistical analysis
Chi-square tests were used for categorical variables, while t-tests were used to examine differences between the variables. P<0.05 was considered statistically significant.
Results
1H NMR spectrum
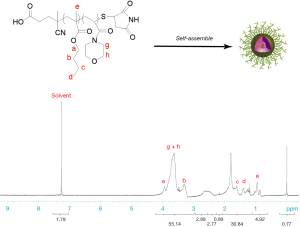
The number-average molecular weight of the copolymer was detected by 1H NMR using CDCl3 as a solvent. In the 1H NMR spectrum of PBMA-b-PNAM, the signals at ~2.2 ppm and ~3.4–3.7 ppm were attributed to the -CH2O and –CH2N protons of the morpholine group indicating that the NAM monomer had been successfully introduced into the chain. It is known that the hydrophobic block was embed in the hydrophobic core of the micelle when the diblock copolymer self-assembled in water, as a result of the, core-shell structure as shown in Figure 1. The structure of the polymer was elucidated by 1H NMR. In the 1H NMR spectrum (Figure 2), the chemical shifts at 1.26 and 1.5 ppm were attributable to the terminal protons of the polymeric side chains, and the chemical shifts at 3.5 and 3.6 can be assigned to the reactions.
Dynamic light scattering and transmission electron microscope
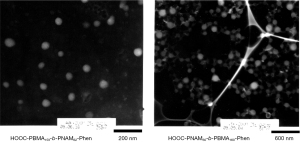
Figure 3 shows the hydrodynamic diameter and size distribution of the micelle-PTX complex obtained by DLS. The two peaks in Figure 3A were the size of the empty PBMA-b-PNAM micelle, the approximately 40 and 235 nm was referring to the HOOC-PBMA-PNAM-Phen (red) and HOOC-PNAM-PBMA-Phen (black), which was similar to the previous study (29). In addition, Figure 3B indicates the size of the micelle entrapped the PTX. The size and size distribution of two micellar systems has little changes, which indicated their stable spherical structure. The size distribution was similar to hydrodynamic diameter as obtained by DLS and clearly confirmed that the micelle-PTX complex was successfully prepared with well-defined core–shell structure. The morphology of the polymeric micelles was determined by DLS. The microstructure of the micelles was investigated by TEM. Figure 4 shows TEM images of the micelles formed by HOOC-PBMA-PNAM-Phen and HOOC-PNAM-PBMA-Phen in aqueous solution. The images were obtained at a copolymer solution concentration of 0.04 mg/mL. The results of TEM and DLS were consistent.

PTX loading profile
Figure 5 shows the PTX loading profile of PBMA-b-PNAM micelles with exchanged hydrophobic and hydrophilic block lengths [HOOC-PBMA-PNAM-Phen (red) and HOOC-PNAM-PBMA-Phen (black)]. Comparing the peaks of PTX obtained in HPLC, it is easily to find that, the PTX loading efficiency of HOOC-PNAM-PBMA-Phen (red) was much lower than that of HOOC-PNAM-PBMA-Phen (black). Based on the interaction between hydrophobic drug and hydrophobic blocks, it is reasonable that the low PTX loading of micellar drug delivery systems of HOOC-PNAM-PBMA-Phen (red) is due to the limited drug retention capacity of the hydrophobic core. In addition, the increasing drug loading level can be realized by increasing the hydrophobicity of the core without changing the micelle size or deforming.
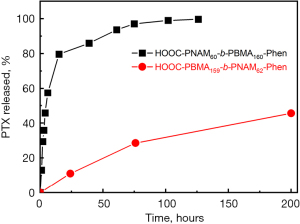
PTX release profiles
Figure 6 shows the PTX release profiles of the PBMA-b-PNAM micelles with different end groups, that is, HOOC-PNAM-PBMA-Phen (red) and HOOC-PNAM-PBMA-Phen (black). It is shown that the PTX release from the micelles with hydrophobic end group on the hydrophobic block was much slower than that of micelle with hydrophilic end group on the hydrophobic block.
Differential scanning calorimeter analysis
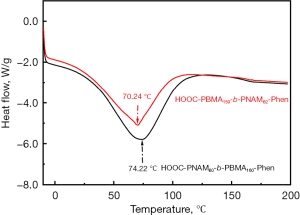
The polymers have glass transition temperature (Tg) values of 70.24 and 74.22 ℃ which was from the HOOC-PBMA-PNAM-Phen (red) and HOOC-PNAM-PBMA-Phen (black) micelles, respectively as shown in Figure 7.
Discussion
For practical application, the release of uncle chemotherapy drugs from the core of micelle system is a key issue (29,33). To control the release of drugs according to the actual clinical needs, many methods, such as micelles and emulsions, have been tried out but they all come up with limited effects, especially on the control of the explosive release of drugs (6). It is known that the loading and release of hydrophobic drug are determined by the hydrophobic blocks via the similar-to-similar interaction. Further analysis shows that the end group on the hydrophobic block plays an important role.
Therefore, in this study, the block copolymers PBMA-b-PNAM with symmetrical and similar block lengths (that is, HOOC-PBMA-PNAM-Phen and HOOC-PNAM-PBMA-Phen) were synthesized by RAFT as shown in Figure 1. Here, the monomers additions process was different for the two copolymers for obtaining the different end group as described in our previous studies (8,24,25).
The PTX release from the micelles with hydrophobic end group on the hydrophobic block was much slower than that of micelle with hydrophilic end group on the hydrophobic block. The reason was shown in the drug loading experiments.
Taken together, the end groups on the hydrophobic blocks inside micellar core strongly dominated the drug loading and release. The nature of the reason was that the hydrophobic similar-to-similar interaction between the hydrophobic drug with the hydrophobic blocks was same as the process mentioned above. Given the illustration is right, the rigid of the core of HOOC-PBMA-PNAM-Phen (red) and HOOC-PNAM-PBMA-Phen (black) micelles should be different, as reflected in the Tg of the micelles. Subsequently, the DSC analysis was used to further analyze the effects of end group on the hydrophobic block on the micelle properties as shown in Figure 6.
The polymeric micelles receive increased attention due to their ability to load therapeutics, deliver the cargo to the site of action, improve the pharmacokinetic of the loaded drug and reduce off-target cytotoxicity (34). However, maintenance of the integrity of the self-assembled system in the circulation and disassembly for drug release at the site of drug action remain a challenge (17). To address this challenge, the drug loading and releasing profile need to be overcome. This study first showed that the hydrophobic end group Phen on the core-forming blocks can promote hydrophobic drug loading and suppress burst release.
The degradation of micelles assembled from the block copolymers with hydrophilic end group on the hydrophobic block (HOOC-PNAM-PBMA-Phen, black) was higher than that of hydrophobic end group on the hydrophobic block (HOOC-PBMA-PNAM-Phen, red), which indicated that the density of the hydrophobic core is looser. In this study, DSC analysis was used to effectively monitor the stability of micelles, which provided insight into the core properties, the segmental rigidity and information of viscosity of micelles.
Conclusions
In the present study, an alternative monomer addition method was successfully applied to tune and obtain two block copolymers: HOOC-PBMA-PNAM-Phen and HOOC-PNAM-PBMA-Phen. Compared with the HOOC-PBMA-PNAM-Phen, the micelles with hydrophobic Phen group on the hydrophobic block (HOOC-PNAM-PBMA-Phen) enhanced drug loading and prolonged drug release, but with a larger particle size. The results indicated that the hydrophobic end group Phen on the core-forming blocks can promote hydrophobic drug loading and suppress burst release.
Acknowledgments
The authors deeply thank Prof Teruo Okano and Prof Masamichi Nakayama for their kind direction and advice. We thank Frank Kitching, MSc., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Funding: The study was funded by the National Natural Science Foundation of China (No. 31470964 and No. 81873215), Program of Shanghai Academic/Technology Research Leader (No. 22XD1404700) and Shanghai Sailing Program (No. 20YF1448500) and Scientific Research Fund of Young Teachers in Naval Military Medical University (No. 2022QN093).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-206/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-206/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-206/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Suh KJ, Ryu MH, Zang DY, et al. AZD8186 in Combination With Paclitaxel in Patients With Advanced Gastric Cancer: Results From a Phase Ib/II Study (KCSG ST18-20). Oncologist 2023;oyad059. [Crossref] [PubMed]
- Fang Z, Lin L, Li Z, et al. Stimuli-responsive heparin-drug conjugates co-assembled into stable nanomedicines for cancer therapy. Acta Biomater 2023;164:422-34. [Crossref] [PubMed]
- Shi W, Wan X, Wang Y, et al. Nanoparticle albumin-bound paclitaxel-based neoadjuvant regimen: A promising treatment option for HER2-low-positive breast cancer. Nanomedicine 2023;49:102666. [Crossref] [PubMed]
- Zhang T, Conrad ED, Gates DP. Di- and tri-block copolymers from the sequential living anionic copolymerization a phosphaalkene with styrene. Polymer 2022;249:124831. [Crossref]
- Qiu X, Ma S, Wang D, et al. The development of multifunctional sulfated polyguluronic acid-based polymeric micelles for anticancer drug delivery. Carbohydr Polym 2023;303:120451. [Crossref] [PubMed]
- Cai W, Lv W, Meng L, et al. The Combined Effect of Nanobubble-IR783-HPPH-Affibody Complex and Laser on HER2-Positive Breast Cancer. Int J Nanomedicine 2023;18:339-51. [Crossref] [PubMed]
- Li W, Li H, Li J, et al. Self-assembled supramolecular nano vesicles for safe and highly efficient gene delivery to solid tumors. Int J Nanomedicine 2012;7:4661-77. [Crossref] [PubMed]
- Zhang F, Hou Y, Zhu M, et al. Death Pathways of Cancer Cells Modulated by Surface Molecule Density on Gold Nanorods. Adv Sci (Weinh) 2021;8:e2102666. [Crossref] [PubMed]
- Zhang F, Zhu X, Gong J, et al. Lysosome-mitochondria-mediated apoptosis specifically evoked in cancer cells induced by gold nanorods. Nanomedicine (Lond) 2016;11:1993-2006. [Crossref] [PubMed]
- Kaditi E, Mountrichas G, Pispas S. Amphiphilic block copolymers by a combination of anionic polymerization and selective post-polymerization functionalization. European Polymer Journal 2011;47:415-34. [Crossref]
- Hari SK, Gauba A, Shrivastava N, et al. Polymeric micelles and cancer therapy: an ingenious multimodal tumor-targeted drug delivery system. Drug Deliv Transl Res 2023;13:135-63. [Crossref] [PubMed]
- Infante P, Malfanti A, Quaglio D, et al. Glabrescione B delivery by self-assembling micelles efficiently inhibits tumor growth in preclinical models of Hedgehog-dependent medulloblastoma. Cancer Lett 2021;499:220-31. [Crossref] [PubMed]
- Deshmukh AS, Chauhan PN, Noolvi MN, et al. Polymeric micelles: Basic research to clinical practice. Int J Pharm 2017;532:249-68. [Crossref] [PubMed]
- Xu DZ, Sun XY, Liang YX, et al. Esterase-Responsive Polymeric Micelles Containing Tetraphenylethene and Poly(ethylene glycol) Moieties for Efficient Doxorubicin Delivery and Tumor Therapy. Bioconjug Chem 2023;34:248-56. [Crossref] [PubMed]
- Luo D, Chen W, Wang W, et al. Low-intensity pulsed ultrasound alleviating myelosuppression of Sprague-Dawley rats after combined treating by paclitaxel and carboplatin. Transl Cancer Res 2021;10:1183-92. [Crossref] [PubMed]
- Batool S, Sohail S, Ud Din F, et al. A detailed insight of the tumor targeting using nanocarrier drug delivery system. Drug Deliv 2023;30:2183815. [Crossref] [PubMed]
- Negut I, Bita B. Polymeric Micellar Systems-A Special Emphasis on "Smart" Drug Delivery. Pharmaceutics 2023;15:976. [Crossref] [PubMed]
- Rijcken CJF, De Lorenzi F, Biancacci I, et al. Design, development and clinical translation of CriPec®-based core-crosslinked polymeric micelles. Adv Drug Deliv Rev 2022;191:114613. [Crossref] [PubMed]
- Li Q, Yao W, Yu X, et al. Drug-loaded pH-responsive polymeric micelles: Simulations and experiments of micelle formation, drug loading and drug release. Colloids Surf B Biointerfaces 2017;158:709-16. [Crossref] [PubMed]
- Tao L, Chan JW, Uhrich KE. Drug loading and release kinetics in polymeric micelles: Comparing dynamic versus unimolecular sugar-based micelles for controlled release. Journal of Bioactive and Compatible Polymers 2015;31:227-41. [Crossref]
- Liu P, Wang B, Weili Qiao JL. Multi-anticancer drugs encapsulated in the micelle: a novel chemotherapy to cancer. Med Hypotheses 2008;71:379-81. [Crossref] [PubMed]
- Jiaying Y, Bo S, Xiaolu W, et al. Arenobufagin-loaded PEG-PLA nanoparticles for reducing toxicity and enhancing cancer therapy. Drug Deliv 2023;30:2177362. [Crossref] [PubMed]
- Li W, Li J, Gao J, et al. The fine-tuning of thermosensitive and degradable polymer micelles for enhancing intracellular uptake and drug release in tumors. Biomaterials 2011;32:3832-44. [Crossref] [PubMed]
- Li W, Zhao H, Qian W, et al. Chemotherapy for gastric cancer by finely tailoring anti-Her2 anchored dual targeting immunomicelles. Biomaterials 2012;33:5349-62. [Crossref] [PubMed]
- Li S, Li F, Wan D, et al. A micelle-based stage-by-stage impelled system for efficient doxorubicin delivery. Bioact Mater 2022;25:783-95. [Crossref] [PubMed]
- Vatansever O, Bahadori F, Bulut S, et al. Coating with cationic inulin enhances the drug release profile and in vitro anticancer activity of lecithin-based nano drug delivery systems. Int J Biol Macromol 2023;237:123955. [Crossref] [PubMed]
- Kim SD, Chakravarti S, Tian J, et al. The phase behavior and the Flory–Huggins interaction parameter of blends containing amorphous poly(resorcinol phthalate-block-carbonate), poly(bisphenol-A carbonate) and poly(ethylene terephthalate). Polymer 2010;51:2199-206. [Crossref]
- Li W, Feng S, Guo Y. Tailoring polymeric micelles to optimize delivery to solid tumors. Nanomedicine (Lond) 2012;7:1235-52. [Crossref] [PubMed]
- Li W, Guo Q, Zhao H, et al. Novel dual-control poly(N-isopropylacrylamide-co-chlorophyllin) nanogels for improving drug release. Nanomedicine (Lond) 2012;7:383-92. [Crossref] [PubMed]
- Li W, Nakayama M, Akimoto J, et al. Effect of block compositions of amphiphilic block copolymers on the physicochemical properties of polymeric micelles. Polymer 2011;52:3783-90. [Crossref]
- Yu J, Chen F, Wang X, et al. Synthesis and characterization of MMP degradable and maleimide cross-linked PEG hydrogels for tissue engineering scaffolds. Polymer Degradation and Stability 2016;133:312-20. [Crossref]
- Guzmán Rodríguez A, Sablón Carrazana M, Rodríguez Tanty C, et al. Smart Polymeric Micelles for Anticancer Hydrophobic Drugs. Cancers (Basel) 2022;15:4. [Crossref] [PubMed]
- Biswas S, Kumari P, Lakhani PM, et al. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 2016;83:184-202. [Crossref] [PubMed]


