
Colorectal cancer in adolescent and young adults: epidemiology in Japan and narrative review
Introduction
In Japan, approximately one million people are diagnosed with cancer annually, 2% of whom are adolescents and young adults (AYAs) (1). Colorectal cancer (CRC) is the most common cancer and the second leading cause of cancer-related death in Japan; more than 150,000 patients are newly diagnosed with CRC, and more than 50,000 patients die from CRC every year (2). Among AYA individuals in Japan, CRC is the fourth most common cancer after breast, uterine, and thyroid cancers (3). The incidence of AYA-CRC is up to 2,000 patients per year, accounting for approximately 10% of all cancers diagnosed in this age group (4). In Japan, healthy AYAs are not recommended for cancer screening, except for cervical cancer (5). Cervical cancer screening with cytology is recommended for women aged 20 years or more, though the implementation rate is reported to be low (6). Regarding CRC, a population-based screening program with annual immunochemical fecal occult blood tests was started in 1992 as a national policy; however, this program only targeted people over 40 years of age based on morbidity (7). Thus, it may be difficult to diagnose AYA-CRC at the asymptomatic stage. In addition, failure to recognize or deny the importance of symptoms may lead to a delayed diagnosis (8). A case-control study showed that the median time from symptom onset to treatment was 217 days in patients aged <50 years compared to 29.5 days in patients aged ≥50 years (9). In this article, we aimed to review the clinicopathological characteristics, genetics, and management of AYA-CRC. Although there have been a lot of excellent review articles about young-onset CRC (10-12), most of them do not focus on the AYA population (15–39 years old). As far as we know, this is the first review article that highlights current situations of AYA-CRC in Japan. We present this article in accordance with the Narrative Review reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-98/rc).
Methods
We conducted an electronic search of articles in the PubMed database published until November 30, 2022. Articles related to AYA-CRC were identified using keywords, “((adolescent and young adult) OR AYA) AND ((colorectal cancer) OR (colon cancer) OR (rectal cancer))”. We also searched and reviewed articles related to young-onset CRC using keywords such as “young-onset AND ((colorectal cancer) OR (colon cancer) OR (rectal cancer))”. Only the articles published in English were included. Articles without abstracts, case reports, and articles without available full text were excluded (Figure 1). When we searched literatures related to young-onset CRC, review articles were also excluded because there was a lot of overlap of information. The entire text of the articles was reviewed and analyzed by AU. No systematic review of the articles or meta-analysis was performed. The detailed methods are summarized in Table 1. Although the definition of AYA varies among reports (13), we used this term for individuals aged 15–39 unless otherwise specified.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1 December 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | #1 “((adolescent and young adult) OR AYA) AND ((colorectal cancer) OR (colon cancer) OR (rectal cancer))”; #2 “young-onset AND ((colorectal cancer) OR (colon cancer) OR (rectal cancer))” |
| Timeframe | Until 30 November 2022 |
| Inclusion and exclusion criteria | Inclusion: English language, abstract, and full-text available. Exclusion: case reports (#1 and #2) and reviews (#2) |
| Selection process | A Ueno reviewed and analyzed the extracted articles |
Epidemiology and clinical characteristics of AYA-CRC
Epidemiology of AYA-CRC in Japan and other countries
The incidence of AYA-CRC in Japan is increasing gradually, from 1,200 patients diagnosed with CRC in 1975 to 2,000 in 2015 (Figure 2A) (4). Increasing trends in AYA-CRC have also been reported in other countries such as the United States (14,15), Canada (16), England (17,18), and Australia (19,20), and similar trends have been observed worldwide (21). The Global Burden of Disease Study has reported the global epidemiology of AYA-CRC; the global incidence of AYA-CRC had increased from 37,285 in 1990 to 76,090 in 2019 (22). Recent changes in lifestyles such as Western-style diet and physical inactivity are thought to have caused the increase of the incidence of AYA-CRC (23). In the United States, one in 1,200 (0.08%) individuals developed CRC at 40 years of age or younger in the current era (24); in particular, the incidence of rectal cancer is increasing more rapidly than colon cancer (25). By 2030, it is estimated that the incidence of CRC in individuals aged 20–34 will increase up to 90% for colon cancer and 124% for rectal cancer (26). On the other hand, the incidence of AYA-CRC in Korea between 2011 and 2015 decreased slightly compared to those between 2006 and 2010 (27). A multinational cohort study in Asia showed that the increasing trend of young-onset (including 40–49 age group) was the most pronounced in male rectal cancer (28).
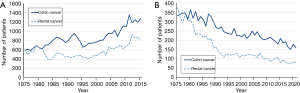
A Japanese multicenter cohort study showed a slight male predominance (56.8%) in the incidence of AYA-CRC with 54.4%, 23.7%, and 21.9% of tumors occurring in the rectum, left colon, and right colon, respectively (29). A single-center study in Taiwan showed that >40% of AYA-CRC cases were stage IV at diagnosis and <10% were stage I at diagnosis (30). The mortality rate of AYA-CRC has decreased in Japan, and the annual number of deaths from CRC has decreased from 700 in 1975 to 250 in 2020 (Figure 2B) (2). In the United States, on the other hand, the mortality of AYA-CRC is still increasing (31,32). Disease stage at diagnosis is the most striking prognostic factor, whereas male sex, black genetic ancestry, no insurance, poorly differentiated histology, higher tumor grade, and exposure to fine particulate matter pollution have also been reported to be associated with worse survival (33-35).
Risk factors for AYA-CRC
Family history of CRC may be an important risk factor for AYA-CRC. Mork et al. retrospectively analyzed 193 patients aged 35 years or younger and found that the family history of CRC in first- and second-degree relatives was present in 11.9% and 32.1%, respectively (36). Another study showed that the presence of a family history of CRC in first-degree relatives increases the risk of CRC by up to 4-fold (37). Understanding the risk factors for AYA-CRC can help identify those who would benefit from CRC screening, and AYAs with a family history may benefit from CRC screening.
According to a recent systematic review, one prospective, five retrospective, and one cross-sectional study investigated the risk factors of young-onset CRC, five of which targeted the AYA population (38). Due to the rare incidence of AYA-CRC, three studies investigated the risk factors for adenomas or other neoplastic polyps and identified family history of CRC, older age, male sex, obesity, less physical activity, smoking, alcohol intake, and diabetes mellitus as risk factors for neoplastic colonic polyps in AYAs (39-41). On the other hand, two studies assessed the risk factors for CRC or rectal cancer in AYAs and revealed that family history of CRC, genetic ancestry other than black or white, and inflammatory bowel disease were associated with a higher frequency of AYA-CRC (42,43). The risk factors and associated evidence are summarized in Table 2. Kim et al. pointed out that several risk factors such as smoking, drinking, and less physical activity can be changed and maintaining good lifestyle habits would help prevent AYA-CRC (39). In a large cohort of female nurses aged 25–42 years, higher intake of vitamin D was associated with decreased risk of early-onset CRC; thus, vitamin D intake may be encouraged in young women (44).
Table 2
| Risk factors | Description | Reference |
|---|---|---|
| Family history | Family history of CRC was associated with an increased risk of NCP (OR, 13.28; 95% CI: 5.70–30.97) | (40,43) |
| Family history of CRC was present in 27% (17/62) of patients ≤30 years old with CRC | ||
| Age | Age over 30 years was associated with an increased risk of colorectal adenoma (OR, 2.37; 95% CI: 1.64–3.42) | (39-41) |
| Higher age was associated with an increased risk of NCP (OR, 1.11; 95% CI: 1.07–1.15) | ||
| Higher age was associated with an increased risk of NCP in young adults aged 30–39 years (OR, 1.09; 95% CI: 1.08–1.11) | ||
| Male sex | Male sex was associated with an increased risk of NCP in young adults aged 30–39 years (OR, 1.37; 95% CI: 1.27–1.49) | (39) |
| Race | Race other than black or white was associated with an increased risk of rectal cancer in AYAs (OR, 1.46; 95% CI: 1.23–1.73) | (42) |
| Obesity | Higher BMI was associated with an increased risk of NCP (OR, 1.07; 95% CI: 1.03–1.12) | (39,40) |
| Obesity was associated with an increased risk of NCP in young adults aged 30–39 years (OR, 1.26; 95% CI: 1.19–1.34) | ||
| Less physical activity | Regular exercise was associated with decreased risk of NCP in young adults aged 30–39 years (OR, 0.89; 95% CI: 0.81–0.97) | (39) |
| Smoking | Current smoking status was associated with an increased risk of colorectal adenoma (OR, 1.48; 95% CI: 1.14–1.91) | (39,41) |
| Current or former smoker was associated with an increased risk of NCP in young adults aged 30–39 years (OR, 1.29; 95% CI: 1.21–1.38) | ||
| Alcohol | Alcohol consumption was associated with an increased risk of colorectal adenoma (OR, 1.29; 95% CI: 1.03–1.63) | (39,41) |
| Alcohol consumption was associated with an increased risk of NCP in young adults aged 30–39 years (OR, 1.25; 95% CI: 1.17–1.33) | ||
| Diabetes mellitus and metabolic syndrome | Diabetes mellitus was associated with an increased risk of NCP (OR, 2.80; 95% CI: 1.06–7.42) | (39,40) |
| Metabolic syndrome was associated with an increased risk of NCP in young adults aged 30–39 years (OR, 1.33; 95% CI: 1.21–1.46). Elevated blood pressure (OR, 1.25; 95% CI: 1.16–1.35) and elevated triglyceride levels (OR, 1.24; 95% CI: 1.16–1.33) were also associated with an increased risk of NCP | ||
| Inflammatory bowel disease | Underlying inflammatory bowel disease was present in 15% (9/62) of patients ≤30 years old with CRC | (43) |
CRC, colorectal cancer; AYA, adolescent and young adult; NCP, neoplastic colonic polyps; OR, odds ratio; CI, confidence interval; BMI, body mass index.
The difference in clinicopathological and molecular characteristics between AYA-CRC and elderly CRC
Several clinicopathological differences have been reported between AYA-CRC and CRC in elderly patients. Adverse histology is more common in AYA-CRC patients than in older patients. In a Japanese multicenter study, 10.2% of resected AYA-CRC cases had mucinous or poorly differentiated histology (29). Another study from the United States revealed that 37% of patients with CRC aged <30 years had poorly differentiated tumors (45). According to the Surveillance, Epidemiology, and End Results database, histopathological diagnoses other than adenocarcinoma were more frequent in patients aged 15–19 (26.7%) than in those aged 35–39 (13.6%) (46). Although most AYA-CRC cases are diagnosed at an advanced stage, the disease-specific and overall survival rates are comparable to or may be better than those of the general population with CRC (17,47).
Regarding genetic and molecular characteristics, Tricoli et al. conducted a comprehensive study using whole-exome sequencing and found that several mutations were significantly more frequent in AYAs with CRC than in patients with CRC aged 61–90 years (48). McVeigh et al. evaluated the genomic profile of AYAs with advanced solid tumors and found that a considerable proportion of patients had targetable mutations (49). Salem et al. compared the molecular profiles of right- and left-sided CRC in AYAs (50). They found that mutations in MSH2 and MSH6, as well as the microsatellite instability (MSI)-high phenotype, were more frequent in right-sided AYA-CRC than in left-sided AYA-CRC (20.8% vs. 4.8%); clinical significance of MSH2/MSH6 mutations and MSI-high phenotype is described in the following section. Chemotherapy regimens for CRC need to be tailored based on the MSI status; for example, adding oxaliplatin to fluoropyrimidine is recommended as adjuvant therapy for MSI-high CRC (51), and immune checkpoint inhibitors are recommended for stage IV MSI-high CRC (52,53). Therefore, MSI testing should be considered for AYAs with CRC, especially those with right-sided tumors. The consensus molecular subtype (CMS) is a recently developed classification system for CRC at the gene expression level (54), and it has been reported that CMS1 was the most common in the AYA age group (55).
Hereditary cancer associated with AYA-CRC
Although most cases of AYA-CRC are sporadic, approximately 30% are thought to have a hereditary component (37,56). While 3–5% of these have well-characterized hereditary cancer syndromes such as Lynch syndrome, familial adenomatous polyposis (FAP) and other rare syndromes including MutYH-associated polyposis, Peutz-Jeghers syndrome, juvenile polyposis, polymerase proofreading-associated polyposis and Cowden/PTEN hamartoma syndrome (Table 3), other hereditary cases remain unexplained (57,58). Patients with these genetic backgrounds tend to develop CRC earlier. In most hereditary CRC syndromes, polyps precede the development of carcinoma, but the exact route to carcinoma seems to differ among the conditions (59). Pearlman et al. have analyzed the prevalence of germline mutations associated with cancer susceptibility among 450 CRC patients younger than 50 years, and they revealed that 16% of patients had genetic cancer susceptibility (60). Thus, genetic testing should be considered for all AYAs with CRC (61), although only a few AYA-CRC patients do not undergo such tests in real-world practice. Details of the two most common syndromes, Lynch syndrome and FAP, are reviewed in this section, and comprehensive descriptions of other syndromes are available in the guidelines of the Japanese Society for Cancer of the Colon and Rectum (58).
Table 3
| Syndrome | Responsible genes | Frequency | Lifetime risk of CRC | Average age at CRC diagnosis (years) | Clinical characteristics |
|---|---|---|---|---|---|
| Lynch syndrome (hereditary nonpolyposis CRC) | MLH1, MSH2, MSH6, PMS2, EPCAM | 2–5% of all CRC | 50–80% | 40–45 | Right-sided, locally advanced tumors |
| Microsatellite instability-high phenotype | |||||
| Commonly associated with various extracolonic cancers | |||||
| FAP | APC on chromosome 5q21 | 1–2% of all CRC (1:10,000–20,000) | Almost 100% | 39 | >100 adenomatous polyposis with average onset before 20 years old |
| Polyposis also found in the stomach and duodenum | |||||
| MutYH-associated polyposis | MutYH | About 1% of all CRC | 35–53% | 35–45 | Broad clinical spectrum sometimes resembling attenuated FAP |
| Peutz-Jeghers syndrome | STK11 | <1% of all CRC (1:25,000–300,000) | 39% by age 70 years | 60 | Commonly associated with extracolonic cancer |
| Melanocytic macules on lips, perioral, and buccal regions | |||||
| Juvenile polyposis syndrome | MADH4 (SMAD4/DPC4), BMPR1A | <1% of all CRC | 17–68% by age 60 years | 35–45 | Hamartomatous polyposis throughout the gastrointestinal tract |
| Polymerase proofreading-associated polyposis | POLE, POLD1 | <1% of all CRC | Reported to be 80% in a small study | 35–40 | Sometimes associated with endometrial, breast, and/or brain tumors |
| Good response to immune checkpoint inhibitors | |||||
| PTEN hamartoma tumor syndrome (Cowden syndrome) | PTEN | <1% of all CRC | 9–16% | 40s | Lifetime risks for a variety of cancers such as breast, thyroid, kidney, and skin cancer |
AYA, adolescent and young adult; CRC, colorectal cancer; FAP, familial adenomatous polyposis.
Lynch syndrome
Lynch syndrome is the most common hereditary syndrome associated with CRC. It is caused by germline mutations in mismatch repair genes, such as MLH1, MSH2, MSH6, and PMS2, and is inherited in an autosomal dominant manner (62). These mismatch repair proteins correct DNA replication errors and they are essential to maintain genetic stability (63). In Lynch syndrome, mismatch repair deficiency (dMMR) accelerates mutation accumulation and increases the risk of carcinogenesis. dMMR-associated cancer usually shows high tumor mutation burden and MSI-high phenotype (63). EPCAM is located upstream of MSH2, and mutations in EPCAM can be another cause of Lynch syndrome. Hypermethylation of MLH1 can cause sporadic dMMR/MSI-high CRC, but this condition is not considered Lynch syndrome. In individuals with Lynch syndrome, the lifetime risk of CRC development is estimated to be 50–80%, and CRC is diagnosed between 40 and 45 years of age on average (37). Two teenage siblings with multiple adenomas and CRC have been reported who had heterozygous variants in PMS2 and POLD1 (57). POLD1 mutations are also known as a cause of hereditary CRC (64); thus, the coexistence of these variants may have accelerated cancer predisposition. The Amsterdam criteria have been used to identify those at risk of Lynch syndrome; however, the sensitivity of these criteria is only 78%; thus, Lynch syndrome should be suspected in young patients with CRC and CRC patients with a history of other cancers, even if they do not meet the Amsterdam criteria (65). The Amsterdam criteria has been revised in 1999 (66); however, its mutation detection rate was not improved enough compared to the original criteria (67). Mutations in BRAF are found in up to 12% of metastatic CRC and BRAF V600E mutations are associated with the female sex, right-sided and advanced CRC, and high mutation burden (68), but these mutations are rare in patients with Lynch syndrome; thus, BRAF-specific immunohistochemistry may be useful to exclude Lynch syndrome (69). Some patients with clinical suspicion of Lynch syndrome and MSI-high tumors lack pathogenic mutations in the mismatch repair gene; these patients are defined as having mutation-negative Lynch syndrome or Lynch-like syndrome (58). Lynch syndrome-related CRC tends to be mucinous, high-grade, and right-sided (70). More than 80% of CRC cases in patients with Lynch syndrome demonstrate an MSI-high phenotype (45). Other cancers associated with Lynch syndrome include endometrial, gastric, small bowel, hepatobiliary, urinary tract, ovarian, and brain tumors. When a brain tumor arises in patients with either Lynch syndrome or FAP, they are considered to have Turcot syndrome (71). Individuals with confirmed or suspected Lynch syndrome should undergo colonoscopy for CRC screening every 1–2 years, beginning at the age of 20–25 years, which has been shown to reduce mortality risk (72,73). Surveillance of other cancers, including uterine, ovarian, gastric, and urinary tract cancers, should also be considered (73,74).
FAP
FAP is the second most common hereditary syndrome associated with AYA-CRC, occurring in 1/17,400 individuals (58). FAP is caused by a loss-of-function mutation in the APC gene. Hundreds to thousands of polyps arise throughout the large bowel and the lifetime risk of CRC approaches 100% in the absence of prophylactic colectomy. The median age for the development of CRC is 35–40 years in these patients (58). Approximately 80% of all causes of death in FAP patients was CRC until the 1980s; however, the proportion has been decreasing to approximately 60% since the 1990s. Among extracolonic manifestations, desmoid tumors and duodenal cancer are the leading causes of death, with incidences of approximately 10% and 6%, respectively (75). Patients with FAP should undergo surveillance with sigmoidoscopy or colonoscopy every 1–2 years beginning at 10–11 years of age (73). Owing to a large number of polyps and the high risk of CRC, patients with classic FAP should undergo prophylactic surgery in their 20s (37,58).
Special consideration in the treatment of AYA-CRC
Chemotherapy regimens
It is essential to test for MSI status before administering chemotherapy to patients with AYA-CRC because they are more likely to have MSI-high tumors, as mentioned in the previous section; MSI-high CRC requires different treatment strategies from microsatellite stable tumors. However, it remains unknown whether the optimal treatment for AYA-CRC differs from that for CRC in elderly patients. Kneuertz et al. reported that young patients (aged <50 years) with CRC received significantly more postoperative chemotherapy but gained only a minimal gain in survival (76). These findings cannot be simply interpreted because MSI status was not assessed in this study, but we should be aware that more intensive treatment may not always be beneficial. In addition, while young patients are less likely to develop severe neutropenia than their older counterparts, the frequency of nausea is higher in young patients (77). For metastatic diseases, no age-related differences in the efficacy of chemotherapy have been reported (78). Nonetheless, all previous studies were limited by their study design or sample size; therefore, further research with an adequate number of patients with AYA, ideally randomized controlled trials, will be required to clarify the optimal treatment for AYA-CRC.
Fertility preservation
Fertility is a significant concern for AYAs diagnosed with cancer. The total fertility rate has been reported to be lower in female CRC survivors than in the general population (79). Adhesions after pelvic surgery, radiotherapy, and systemic chemotherapy can cause infertility. Chemoradiotherapy for rectal cancer is associated with a high risk of infertility in female patients, whereas most standard chemotherapy regimens are associated with an intermediate-to-low risk (80). The risk of chemotherapy-induced infertility seems to differ according to the chemotherapy regimen; 5-fluorouracil (5-FU) is believed to cause a temporary reduction in sperm count in men but has a low risk of causing amenorrhea in female patients, whereas oxaliplatin has been suggested to have moderate gonadal toxicity (81). Various fertility preservation options can be offered to AYA patients with cancer. The Japan Society of Clinical Oncology has published clinical practice guidelines for fertility preservation that recommend embryo (fertilized oocyte) cryopreservation for female patients with a male partner (grade B), unfertilized oocyte cryopreservation for female patients without a partner (grade C1), ovarian tissue cryopreservation for female patients who require urgent cancer-directed therapy or in whom ovulation induction is difficult for oocyte harvesting (grade C1), and sperm cryopreservation for male patients (grade B) (82). Because up to 88% of male patients experience sexual dysfunction after surgery for rectal cancer (83), nerve-sparing surgery is recommended when there is a risk of erectile or ejaculatory dysfunction (82). The role of fertility preservation should be informed to patients before surgery, pelvic radiation, and/or chemotherapy (74). However, in a case series from the United States, only 20% of young women (aged 18–45 years) with CRC received fertility counseling (84). In a recent international cross-sectional study, approximately half of the young patients with CRC (aged <50 years) discussed reproductive health with their healthcare providers (85). In Japan, fertility preservation is often provided for patients with breast or hematologic cancer, but rarely for patients with CRC (86). Physicians who treat AYA-CRC patients should also explain fertility preservation to patients before treatment.
Survivorship
Owing to the increased incidence and improved mortality of AYA-CRC, the number of young CRC survivors is likely to increase in the coming decades. Survivors are at risk of CRC recurrence, second primary cancers, and the long-term adverse effects of CRC and anticancer treatment and may suffer from psychological, reproductive, genetic, social, and employment concerns. Thus, special attention is needed for AYA-CRC survivors who have completed active anticancer treatment (87). Previous studies have shown that AYA-CRC survivors (≥2 years after diagnosis) have a 2.83-fold (95% confidence interval: 2.23–3.59) higher risk of hospitalization than their siblings or matched general population (88). Because approximately 20% of patients with AYA-CRC develop a secondary primary neoplasm within 35 years, surveillance for a second primary malignancy may be required and the second cancer tends to be diagnosed within five years after the primary cancer diagnosis (89-91). CRC is the second most common cancer after AYA-CRC, followed by cervical and thyroid cancers (89). On the other hand, stomach, liver and bile, and pancreas cancers were reported to be leading causes of secondary cancer-related deaths (92). In addition, it has been reported that the health-related quality of life of AYAs with CRC is low, even in nonmetastatic patients (93). Returning to work is sometimes challenging for CRC survivors (94,95), and a large questionnaire-based study revealed that 25% of young cancer survivors were unemployed (96). Psychosocial support and networking through AYA patient communities will play an important role because these patients may have limited life experiences and underdeveloped coping skills (97). Especially, male patients aged <50 years were reported to have a higher risk of mental health disorders after diagnosis of CRC compared to average-age CRC patients (98). Several studies on survivorship models for patients with AYA-CRC have been reported; however, evidence of their effectiveness is lacking (99). Moreover, most phase III therapeutic trials of AYAs in cancer have not included patient-reported outcomes (100). Future studies focusing on the quality of life of AYA-CRC survivors are warranted.
Future perspectives for early diagnosis of AYA-CRC
Educational strategies are needed to increase the awareness of patients, primary care physicians, and gastroenterologists to reduce the delayed diagnosis of AYA-CRC. First, AYAs should be informed that they can develop CRC and that the initial symptoms may be rectal bleeding, weight loss, changes in bowel habits, abdominal pain, and/or iron deficiency anemia (74,101,102). Second, all physicians should consider screening for CRC in all AYAs complaining of the above symptoms, especially if they have a family history of CRC (74). Finally, early detection of CRC in asymptomatic AYAs remains a challenging issue because population-based CRC surveillance for AYAs has not been justified in terms of cost-effectiveness. However, every AYA in Japan can undergo individual opportunistic cancer screening with out-of-pocket payment. Increased AYA-CRC awareness may encourage AYAs to engage in out-of-pocket CRC surveillance, as reported for breast cancer screening (103). Kwak et al. prospectively evaluated the prevalence and characteristics of colorectal adenomas in 4,286 asymptomatic young adults (aged 20–39 years) and found that 0.9% of the participants had advanced adenomas (41). In particular, individuals aged >30 years, current smokers, and alcohol drinkers had a significantly higher risk of developing adenomas. In a recent cross-sectional study, the prevalence of colorectal neoplasia in AYAs who underwent colonoscopic screening was 14.9% (104). This information will help AYAs decide whether to perform out-of-pocket CRC surveillance to avoid AYA-CRC-related deaths. We found that only a few studies have been reported from Japan; thus, further research is warranted.
Conclusions
The incidence of AYA-CRC has increased in Japan and other countries. The diagnosis of AYA-CRC is often delayed in current practice; thus, it is necessary for patients, primary care physicians, and gastroenterologists to consider and check for malignant disease, even in AYA patients. AYA-CRC tends to have an unfavorable histology and more advanced disease at diagnosis than CRC in older patients. Up to 5% of AYA-CRC cases are associated with well-described hereditary cancer syndromes; therefore, genetic testing should be considered for all AYAs with CRC. Fertility preservation and survivorship are also important, and adequate explanations and care should be provided to patients with AYA-CRC before and after treatment.
Acknowledgments
We would like to thank Editage (www.editage.com) for language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-98/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-98/prf
Conflicts of Interest: All the authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-98/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Katanoda K, Shibata A, Matsuda T, et al. Childhood, adolescent and young adult cancer incidence in Japan in 2009-2011. Jpn J Clin Oncol 2017;47:762-71. [Crossref] [PubMed]
- Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare). Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html. Accessed Feb 7, 2023.
- Nakata K, Hiyama E, Katanoda K, et al. Cancer in adolescents and young adults in Japan: epidemiology and cancer strategy. Int J Clin Oncol 2022;27:7-15. [Crossref] [PubMed]
- Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2015;45:884-91. [Crossref] [PubMed]
- Goto R, Hamashima C, Mun S, et al. Why screening rates vary between Korea and Japan--differences between two national healthcare systems. Asian Pac J Cancer Prev 2015;16:395-400. [Crossref] [PubMed]
- Saitoh E, Saika K, Morisada T, et al. Status of cervical cancer screening among adolescents and young adults (AYA) in Japan. Int J Clin Oncol 2022;27:473-80. [Crossref] [PubMed]
- Saito Y, Oka S, Kawamura T, et al. Colonoscopy screening and surveillance guidelines. Dig Endosc 2021;33:486-519. [Crossref] [PubMed]
- Barr RD, Ferrari A, Ries L, et al. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr 2016;170:495-501. [Crossref] [PubMed]
- Scott RB, Rangel LE, Osler TM, et al. Rectal cancer in patients under the age of 50 years: the delayed diagnosis. Am J Surg 2016;211:1014-8. [Crossref] [PubMed]
- Sinicrope FA. Increasing Incidence of Early-Onset Colorectal Cancer. N Engl J Med 2022;386:1547-58. [Crossref] [PubMed]
- Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020;158:341-53. [Crossref] [PubMed]
- Eng C, Jácome AA, Agarwal R, et al. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol 2022;23:e116-28. [Crossref] [PubMed]
- Laurence V, Marples M, Stark DP. Adult Cancers in Adolescents and Young Adults. Prog Tumor Res 2016;43:64-73. [Crossref] [PubMed]
- Kasi PM, Shahjehan F, Cochuyt JJ, et al. Rising Proportion of Young Individuals With Rectal and Colon Cancer. Clin Colorectal Cancer 2019;18:e87-95. [Crossref] [PubMed]
- Sheth Bhutada J, Hwang A, Liu L, et al. Poor-Prognosis Metastatic Cancers in Adolescents and Young Adults: Incidence Patterns, Trends, and Disparities. JNCI Cancer Spectr 2021;5:pkab039. [Crossref] [PubMed]
- O'Sullivan DE, Hilsden RJ, Ruan Y, et al. The incidence of young-onset colorectal cancer in Canada continues to increase. Cancer Epidemiol 2020;69:101828. [Crossref] [PubMed]
- Franklyn J, Lomax J, Labib PLZ, et al. Young-onset colorectal cancer: Insights from an English population-based study. Colorectal Dis 2022;24:1063-72. [Crossref] [PubMed]
- Chambers AC, Dixon SW, White P, et al. Demographic trends in the incidence of young-onset colorectal cancer: a population-based study. Br J Surg 2020;107:595-605. [Crossref] [PubMed]
- Haggar FA, Preen DB, Pereira G, et al. Cancer incidence and mortality trends in Australian adolescents and young adults, 1982-2007. BMC Cancer 2012;12:151. [Crossref] [PubMed]
- Troeung L, Sodhi-Berry N, Martini A, et al. Increasing Incidence of Colorectal Cancer in Adolescents and Young Adults Aged 15-39 Years in Western Australia 1982-2007: Examination of Colonoscopy History. Front Public Health 2017;5:179. [Crossref] [PubMed]
- Lui RN, Tsoi KKF, Ho JMW, et al. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol Biomarkers Prev 2019;28:1275-82. [Crossref] [PubMed]
- Pan H, Zhao Z, Deng Y, et al. The global, regional, and national early-onset colorectal cancer burden and trends from 1990 to 2019: results from the Global Burden of Disease Study 2019. BMC Public Health 2022;22:1896. [Crossref] [PubMed]
- Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol 2021;18:230-43. [Crossref] [PubMed]
- Hubbard JM, Grothey A. Adolescent and young adult colorectal cancer. J Natl Compr Canc Netw 2013;11:1219-25. [Crossref] [PubMed]
- O'Connell JB, Maggard MA, Liu JH, et al. Rates of colon and rectal cancers are increasing in young adults. Am Surg 2003;69:866-72. [Crossref] [PubMed]
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150:17-22. [Crossref] [PubMed]
- Hur H, Oh CM, Won YJ, et al. Characteristics and Survival of Korean Patients With Colorectal Cancer Based on Data From the Korea Central Cancer Registry Data. Ann Coloproctol 2018;34:212-21. [Crossref] [PubMed]
- Sung JJY, Chiu HM, Jung KW, et al. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol 2019;114:322-9. [Crossref] [PubMed]
- Nakayama Y, Kobayashi H, Kawamura H, et al. The long-term outcomes in adolescent and young adult patients with colorectal cancer -A multicenter large-scale cohort study. J Cancer 2020;11:3180-5. [Crossref] [PubMed]
- Chou CL, Chang SC, Lin TC, et al. Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. Am J Surg 2011;202:574-82. [Crossref] [PubMed]
- Lewis DR, Siembida EJ, Seibel NL, et al. Survival outcomes for cancer types with the highest death rates for adolescents and young adults, 1975-2016. Cancer 2021;127:4277-86. [Crossref] [PubMed]
- Anderson C, Nichols HB. Trends in Late Mortality Among Adolescent and Young Adult Cancer Survivors. J Natl Cancer Inst 2020;112:994-1002. [Crossref] [PubMed]
- Teng A, Lee DY, Cai J, et al. Patterns and outcomes of colorectal cancer in adolescents and young adults. J Surg Res 2016;205:19-27. [Crossref] [PubMed]
- Lee DY, Teng A, Pedersen RC, et al. Racial and Socioeconomic Treatment Disparities in Adolescents and Young Adults with Stage II-III Rectal Cancer. Ann Surg Oncol 2017;24:311-8. [Crossref] [PubMed]
- Ou JY, Hanson HA, Ramsay JM, et al. Fine Particulate Matter Air Pollution and Mortality among Pediatric, Adolescent, and Young Adult Cancer Patients. Cancer Epidemiol Biomarkers Prev 2020;29:1929-39. [Crossref] [PubMed]
- Mork ME, You YN, Ying J, et al. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J Clin Oncol 2015;33:3544-9. [Crossref] [PubMed]
- Connell LC, Mota JM, Braghiroli MI, et al. The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr Treat Options Oncol 2017;18:23. [Crossref] [PubMed]
- Christodoulides N, Lami M, Malietzis G, et al. Sporadic colorectal cancer in adolescents and young adults: a scoping review of a growing healthcare concern. Int J Colorectal Dis 2020;35:1413-21. [Crossref] [PubMed]
- Kim NH, Jung YS, Yang HJ, et al. Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20-39 Years Old). Clin Gastroenterol Hepatol 2019;17:115-22. [Crossref] [PubMed]
- Chen KC, Chung CS, Hsu WF, et al. Identification of risk factors for neoplastic colonic polyps in young adults with bloody stool in comparison with those without symptom. J Gastroenterol Hepatol 2018;33:1335-40. [Crossref] [PubMed]
- Kwak JY, Kim KM, Yang HJ, et al. Prevalence of colorectal adenomas in asymptomatic young adults: a window to early intervention? Scand J Gastroenterol 2016;51:731-8. [Crossref] [PubMed]
- Tawadros PS, Paquette IM, Hanly AM, et al. Adenocarcinoma of the rectum in patients under age 40 is increasing: impact of signet-ring cell histology. Dis Colon Rectum 2015;58:474-8. [Crossref] [PubMed]
- Al-Barrak J, Gill S. Presentation and outcomes of patients aged 30 years and younger with colorectal cancer: a 20-year retrospective review. Med Oncol 2011;28:1058-61. [Crossref] [PubMed]
- Kim H, Lipsyc-Sharf M, Zong X, et al. Total Vitamin D Intake and Risks of Early-Onset Colorectal Cancer and Precursors. Gastroenterology 2021;161:1208-1217.e9. [Crossref] [PubMed]
- Tricoli JV, Seibel NL, Blair DG, et al. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J Natl Cancer Inst 2011;103:628-35. [Crossref] [PubMed]
- Holowatyj AN, Lewis MA, Pannier ST, et al. Clinicopathologic and Racial/Ethnic Differences of Colorectal Cancer Among Adolescents and Young Adults. Clin Transl Gastroenterol 2019;10:e00059. [Crossref] [PubMed]
- Lipsyc-Sharf M, Zhang S, Ou FS, et al. Survival in Young-Onset Metastatic Colorectal Cancer: Findings From Cancer and Leukemia Group B (Alliance)/SWOG 80405. J Natl Cancer Inst 2022;114:427-35. [Crossref] [PubMed]
- Tricoli JV, Boardman LA, Patidar R, et al. A mutational comparison of adult and adolescent and young adult (AYA) colon cancer. Cancer 2018;124:1070-82. [Crossref] [PubMed]
- McVeigh TP, Sundar R, Diamantis N, et al. The role of genomic profiling in adolescents and young adults (AYAs) with advanced cancer participating in phase I clinical trials. Eur J Cancer 2018;95:20-9. [Crossref] [PubMed]
- Salem ME, Battaglin F, Goldberg RM, et al. Molecular Analyses of Left- and Right-Sided Tumors in Adolescents and Young Adults with Colorectal Cancer. Oncologist 2020;25:404-13. [Crossref] [PubMed]
- Cohen R, Taieb J, Fiskum J, et al. Microsatellite Instability in Patients With Stage III Colon Cancer Receiving Fluoropyrimidine With or Without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J Clin Oncol 2021;39:642-51. [Crossref] [PubMed]
- Lenz HJ, Van Cutsem E, Luisa Limon M, et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol 2022;40:161-70. [Crossref] [PubMed]
- André T, Shiu KK, Kim TW, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 2020;383:2207-18. [Crossref] [PubMed]
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350-6. [Crossref] [PubMed]
- Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019;125:2002-10. [Crossref] [PubMed]
- Staal DP, Vlooswijk C, Mols F, et al. Diagnosed with a common cancer at an unusual age: causal attributions of survivors of adolescent and young adult colorectal cancer. Support Care Cancer 2021;29:409-16. [Crossref] [PubMed]
- Schamschula E, Kinzel M, Wernstedt A, et al. Teenage-Onset Colorectal Cancers in a Digenic Cancer Predisposition Syndrome Provide Clues for the Interaction between Mismatch Repair and Polymerase δ Proofreading Deficiency in Tumorigenesis. Biomolecules 2022;12:1350. [Crossref] [PubMed]
- Tomita N, Ishida H, Tanakaya K, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2020 for the Clinical Practice of Hereditary Colorectal Cancer. Int J Clin Oncol 2021;26:1353-419. [Crossref] [PubMed]
- Ma H, Brosens LAA, Offerhaus GJA, et al. Pathology and genetics of hereditary colorectal cancer. Pathology 2018;50:49-59. [Crossref] [PubMed]
- Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol 2017;3:464-71. [Crossref] [PubMed]
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 2015;17:70-87. [Crossref] [PubMed]
- Peltomäki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol 2003;21:1174-9. [Crossref] [PubMed]
- Eso Y, Shimizu T, Takeda H, et al. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 2020;55:15-26. [Crossref] [PubMed]
- Bellido F, Pineda M, Aiza G, et al. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: review of reported cases and recommendations for genetic testing and surveillance. Genet Med 2016;18:325-32. [Crossref] [PubMed]
- Durno C, Aronson M, Bapat B, et al. Family history and molecular features of children, adolescents, and young adults with colorectal carcinoma. Gut 2005;54:1146-50. [Crossref] [PubMed]
- Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. [Crossref] [PubMed]
- Park JG, Vasen HF, Park YJ, et al. Suspected HNPCC and Amsterdam criteria II: evaluation of mutation detection rate, an international collaborative study. Int J Colorectal Dis 2002;17:109-14. [Crossref] [PubMed]
- Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol 2021;32:959-67. [Crossref] [PubMed]
- Capper D, Voigt A, Bozukova G, et al. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int J Cancer 2013;133:1624-30. [Crossref] [PubMed]
- Levine O, Zbuk K. Colorectal cancer in adolescents and young adults: Defining a growing threat. Pediatr Blood Cancer 2019;66:e27941. [Crossref] [PubMed]
- Ganapathy A, Diaz EJ, Coleman JT, et al. Tumor Syndromes: Neurosurgical Evaluation and Management. Neurosurg Clin N Am 2022;33:91-104. [Crossref] [PubMed]
- Barrow P, Khan M, Lalloo F, et al. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg 2013;100:1719-31. [Crossref] [PubMed]
- Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol 2015;33:209-17. [Crossref] [PubMed]
- Boardman LA, Vilar E, You YN, et al. AGA Clinical Practice Update on Young Adult-Onset Colorectal Cancer Diagnosis and Management: Expert Review. Clin Gastroenterol Hepatol 2020;18:2415-24. [Crossref] [PubMed]
- Iwama T, Tamura K, Morita T, et al. A clinical overview of familial adenomatous polyposis derived from the database of the Polyposis Registry of Japan. Int J Clin Oncol 2004;9:308-16. [Crossref] [PubMed]
- Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg 2015;150:402-9. [Crossref] [PubMed]
- Hubbard J, Thomas DM, Yothers G, et al. Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set. J Clin Oncol 2012;30:2334-9. [Crossref] [PubMed]
- Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 2000;321:531-5. [Crossref] [PubMed]
- Stupart D, Win AK, Winship IM, et al. Fertility after young-onset colorectal cancer: a study of subjects with Lynch syndrome. Colorectal Dis 2015;17:787-93. [Crossref] [PubMed]
- Oktay K, Harvey BE, Partridge AH, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:1994-2001. [Crossref] [PubMed]
- Marhhom E, Cohen I. Fertility preservation options for women with malignancies. Obstet Gynecol Surv 2007;62:58-72. [Crossref] [PubMed]
- Tozawa A, Kimura F, Takai Y, et al. Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for fertility preservation in childhood, adolescent, and young adult cancer patients: part 2. Int J Clin Oncol 2022;27:281-300. [Crossref] [PubMed]
- Traa MJ, De Vries J, Roukema JA, et al. Sexual (dys)function and the quality of sexual life in patients with colorectal cancer: a systematic review. Ann Oncol 2012;23:19-27. [Crossref] [PubMed]
- Strong M, Peche W, Scaife C. Incidence of fertility counseling of women of child-bearing age before treatment for colorectal cancer. Am J Surg 2007;194:765-7; discussion 767-8. [Crossref] [PubMed]
- Araujo L, Rebic N, Dau H, et al. Reproductive Health Experiences of Females Diagnosed with Young-Onset Colorectal Cancer: A Multi-Method Cross-Sectional Survey. Curr Oncol 2022;29:465-78. [Crossref] [PubMed]
- Takae S, Kato K, Watanabe C, et al. A practical survey of fertility-preservation treatments in the startup phase in Japan. J Obstet Gynaecol Res 2022;48:1061-75. [Crossref] [PubMed]
- El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin 2015;65:428-55. [Crossref] [PubMed]
- Anderson C, Kaddas HK, Ou JY, et al. Hospitalization after Adolescent and Young Adult (AYA) Cancer: A Population-Based Study in Utah. Cancer Epidemiol Biomarkers Prev 2020;29:336-42. [Crossref] [PubMed]
- Goldfarb M, Rosenberg AS, Li Q, et al. Impact of latency time on survival for adolescents and young adults with a second primary malignancy. Cancer 2018;124:1260-8. [Crossref] [PubMed]
- Trama A, Tittarelli A, Barigelletti G, et al. Excess risk of subsequent malignant neoplasms in adolescent and young adult cancer survivors: Results from the first Italian population-based cohort. Cancer 2022;128:364-72. [Crossref] [PubMed]
- Bright CJ, Reulen RC, Winter DL, et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol 2019;20:531-45. [Crossref] [PubMed]
- He X, Wu W, Ding Y, et al. Excessive risk of second primary cancers in young-onset colorectal cancer survivors. Cancer Med 2018;7:1201-10. [Crossref] [PubMed]
- Miller KA, Stal J, Gallagher P, et al. Time from Diagnosis and Correlates of Health-Related Quality of Life among Young Adult Colorectal Cancer Survivors. Cancers (Basel) 2021;13:4045. [Crossref] [PubMed]
- Lim CYS, Laidsaar-Powell RC, Young JM, et al. Work: saviour or struggle? A qualitative study examining employment and finances in colorectal cancer survivors living with advanced cancer. Support Care Cancer 2022;30:9057-69. [Crossref] [PubMed]
- Gruß I, Hanson G, Bradley C, et al. Colorectal cancer survivors' challenges to returning to work: A qualitative study. Eur J Cancer Care (Engl) 2019;28:e13044. [Crossref] [PubMed]
- Dahl AA, Fosså SD, Lie HC, et al. Employment Status and Work Ability in Long-Term Young Adult Cancer Survivors. J Adolesc Young Adult Oncol 2019;8:304-11. [Crossref] [PubMed]
- Bailey CE, Tran Cao HS, Hu CY, et al. Functional deficits and symptoms of long-term survivors of colorectal cancer treated by multimodality therapy differ by age at diagnosis. J Gastrointest Surg 2015;19:180-8; discussio 188.
- Howren A, Sayre EC, Cheng V, et al. Risk of Anxiety and Depression after Diagnosis of Young-Onset Colorectal Cancer: A Population-Based Cohort Study. Curr Oncol 2022;29:3072-81. [Crossref] [PubMed]
- Ke Y, Ng T, Chan A. Survivorship care models for breast cancer, colorectal cancer, and adolescent and young adult (AYA) cancer survivors: a systematic review. Support Care Cancer 2018;26:2125-41. [Crossref] [PubMed]
- Berkman AM, Murphy KM, Siembida EJ, et al. Inclusion of Patient-Reported Outcomes in Adolescent and Young Adult Phase III Therapeutic Trials: An Analysis of Cancer Clinical Trials Registered on ClinicalTrials.gov. Value Health 2021;24:1820-7. [Crossref] [PubMed]
- Rajagopalan A, Antoniou E, Morkos M, et al. Is colorectal cancer associated with altered bowel habits in young patients? ANZ J Surg 2021;91:943-6. [Crossref] [PubMed]
- Demb J, Liu L, Murphy CC, et al. Young-onset colorectal cancer risk among individuals with iron-deficiency anaemia and haematochezia. Gut 2020; Epub ahead of print. [Crossref] [PubMed]
- Kwok C, Lee MJ, Lee CF. Breast Cancer Perceptions and Screening Behaviours Among Korean Women in Australia. J Immigr Minor Health 2020;22:126-33. [Crossref] [PubMed]
- Kim I, Lee HH, Ko YJ, et al. Factors associated with the risk of colorectal neoplasia in young adults under age 40. Korean J Intern Med 2022;37:969-78. [Crossref] [PubMed]



