
Real-world treatment patterns and survival outcomes for treated biliary tract cancer patients using administrative databases in Ontario
Highlight box
Key findings
• Between January 2010 and December 2019, 2,142 patients were diagnosed and received a treatment (usually gem/cis) for a biliary tract cancer (BTC) in Ontario.
• Median overall survival was 11.0 months from diagnosis and 7.4 months from initiation of systemic therapy.
What is known and what is new?
• Previous studies that examined the incidence and mortality rates of BTCs in Canada are outdated and lack specific treatment and clinical outcomes.
• This manuscript aims to identify Ontario-specific survival outcomes, as well as treatment patterns, in patients with BTCs.
What is the implication, and what should change now?
• BTC patients have a low survival probability and there is a large unmet need for new therapies.
• Future studies should report the changes in treatment patterns and the impact on survival, HCRU and costs as these new therapies are made available.
Introduction
Biliary tract cancers (BTCs) comprise of a diverse group of malignancies including cancers of the gallbladder (GBC), intra- and extrahepatic cholangiocarcinoma (IHCC and EHCC, respectively), and in some series ampulla of Vater (AoV) tumors (1). In many publicly available Canadian datasets, BTCs have historically been grouped together with hepatocellular carcinoma (HCC) as ‘liver cancer’. Thus, it has been difficult to understand trends in incidence and mortality by specific disease sites, which reflects the need for more informative datasets (2).
Data for all BTCs subtypes in Canada is limited, however, a few retrospective analyses are available. Xiao and colleagues conducted a retrospective analysis using population-based cancer registries from 1992 to 2010 to look at the incidence and mortality of GBC and EHCC in Canada (3). Results of the study found the national crude and age-standardized incidence rate (ASIR) of GBC and EHCC was 30.92 and 21.14 cases per million individuals per year, respectively (3). In another study, Flemming et al. conducted a retrospective, population-based cohort study of all patients diagnosed with incident BTC in Ontario from 1994 through 2012 (2). They found that the ASIR for all BTC cases increased from 34.7 per 100,000 person-years in 1994 to 42.7 per 100,000 person-years in 2012, representing an average increase of 1.6% per year (2). Another analysis by Cancer Care Ontario (CCO) found that BTCs accounted for 3,351 new cancer cases and 923 deaths between 2012 and 2016 which, when standardized to the age distribution of the 2011 Canadian population, translates to an incidence and mortality rate of 5.6 and 1.5 per 100,000 population, respectively (4). In the early stages, surgical resection and adjuvant systemic therapy or chemoradiation are the most effective treatments for BTCs. However, most patients are diagnosed at locally advanced or metastatic disease stage due to the late manifestation and non-specific nature of clinical symptoms (5). For these patients, palliative systemic therapy is the only option (6).
Over a decade ago, the ABC-02 trial established gemcitabine plus cisplatin (gem/cis) as the standard of care for advanced BTCs (7). Other common treatments include gemcitabine monotherapy (gemmono) or gemcitabine with an alternative platinum compound being used when patients are cisplatin ineligible (6). There have been no improvements in systemic therapy for BTCs until recently when the TOPAZ trial showed that adding durvalumab to gemcitabine and cisplatin improved overall survival (OS) in advanced BTCs (8). In 2016, the 5-year survival estimates for all BTCs in Ontario was 19.3%, which is similar to the 5-year survival estimates for only GBC across Canada (4,9). Despite some published studies, information on Canadian-specific survival outcomes and treatment patterns in BTCs and by specific subtypes of BTCs are lacking. Therefore, we sought to identify Canadian-specific survival outcomes, as well as treatment patterns, in patients diagnosed with BTCs between January 2010 and December 2019 using real-world, population-level data from Ontario databases. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-155/rc).
Methods
Study design
Patients included in this population were men and women, with a valid Ontario Health Insurance Plan (OHIP), who were diagnosed with a BTC between January 1, 2010 and December 31, 2019 based on the following ICD-10 codes: C22.1—intrahepatic bile duct carcinoma (IHCC), C23.9—malignant neoplasm of gallbladder (GBC), C24.0—malignant neoplasm of extrahepatic bile duct (EHCC), C24.1—malignant neoplasm of AoV, C24.8—malignant neoplasm of overlapping sites of biliary tract, and C24.9—malignant neoplasm of biliary tract, unspecified.
Patients were excluded if they were under the age of 18 years or over 105 years of age, had another cancer diagnosis any time prior to their BTC diagnosis (i.e., BTC had to be their first cancer diagnosis) or were diagnosed with late-stage (stage IV) BTC but had surgical resection. Patients were also excluded if they received a first-line (1L) treatment not intended for BTCs (e.g., anastrozole, vinorelbine, etc.) or if they were an early-stage patient (stage I–III, unknown/missing) who did not receive a treatment from a pre-defined list of BTC treatments as outlined in Table 1.
Table 1
| Treatment name | Description |
|---|---|
| Gem/carb | Gemcitabine in combination with carboplatin |
| Gem/cis | Gemcitabine in combination with cisplatin |
| Fol | FOLFOX, FOLFIRI, FOLFIRINOX, and related regimens |
| Gemtaxane | Gemcitabine in combination with a taxane (i.e., nab-paclitaxel) |
| Gemmono | Gemcitabine monotherapy |
| Other | Capecitabine monotherapy, capecitabine with gemcitabine, carboplatin with a taxane, fluorouracil monotherapy, fluorouracil with gemcitabine and fluorouracil with cisplatin |
| Nonbtc | Any non-BTC specific regimens that are not included in the above categories |
FOLFOX, 5-FU, FA, and oxaliplatin; FOLFIRI, 5-FU, FA, and irinotecan; FOLFIRINOX, 5-FU, FA, irinotecan, and oxaliplatin; 5-FU, 5-fluorouracil; FA, folinic acid; BTC, biliary tract cancer.
Patients were classified as either those who were initially diagnosed with early-stage disease (stages I, II, III, unknown/missing) then developed recurrence (recurrent) or those who were diagnosed with unresectable late-stage (stage IV) disease at presentation (de novo). Since the Institute for Clinical Evaluative Sciences (ICES) data does not have staging information aside from stage at diagnosis, it was not possible to identify if and when patients recurred. For those patients who were initially diagnosed with stage I–III BTCs, treatment with gemcitabine plus a platinum-based agent (e.g., cisplatin, oxaliplatin, carboplatin) was used as a proxy to determine progression and/or recurrence events. Gemcitabine plus platinum-based agent(s) were assumed to be 1L treatment in the metastatic setting. For AoV patients, progression or recurrence for early-stage patients was defined as, receiving FOLFOX [5-fluorouracil (5-FU), folinic acid (FA), and oxaliplatin] or FOLFIRI (5-FU, FA, and irinotecan). These therapies determined recurrence based on input from the Canadian external experts.
1L treatments only included regimens such as gemcitabine in combination with carboplatin (gem/carb), gem/cis, FOLFOX, FOLFIRI, FOLFIRINOX (5-FU, FA, irinotecan, and oxaliplatin), and related regimens (fol) and gemcitabine in combination with a taxane (gemtaxane). Patients who received nonbtc (any non-BTC specific regimens that are not included in the above categories) treatment in 1L were excluded from this study. Patients who received gemmono and other treatment in 1L were included in the study but these two regimens were removed from their treatment line sequencing, the rationale for which is explained below. Other and nonbtc were possible therapy groups in the second-line (2L) setting.
Only BTC-specific systemic therapies were included in this study. All endocrine drugs, supportive therapies and clinical trial drugs were excluded from treatment line sequencing. Similarly, regimens including platinum drugs (i.e., cisplatin), which did not include gemcitabine (i.e., cisplatin with vinorelbine) were also removed from treatment line sequencing.
Gemmono and other treatment only were excluded from 1L treatment sequencing; accordingly, the patients who received these treatments in 1L were not removed from the study entirely. This was done to remove potential patients receiving adjuvant gemcitabine; adjuvant gemcitabine monotherapy use in Canada was common during the 1st half of the study period until adjuvant capecitabine from the randomized phase III BILCAP study became the standard of care (10). Patients who received gemmono or other treatment in 1L and received either gem/carb, gem/cis, fol or gemtaxane in 2L were assigned to 1L treatment categories based on their 2L treatment. For example, if 100 patients who received gemmono in 1L went on to receive gem/cis in 2L, gemmono would be removed from their treatment line sequencing and those 100 patients would be assigned to the gem/cis group in 1L. Patients who received gemmono or other treatment in 1L and went on to receive no subsequent treatment were removed from this study to remove the potential of including patients who only received adjuvant gemmono and capecitabine. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data sources
Data were accessed through ICES, which collects data on public coverage via the OHIP and other population-level health information. To determine the trajectory of care over time, health information on each individual patient was linked to applicable datasets. The following datasets were used: Activity Level Reporting (ALR), Continuing Care Reporting System (CCRS), Care Providers Database (CPDB), Home Care Database (HCD), Hospital Discharge Abstract Database (DAD), Local Health Integration Network (LHIN), National Ambulatory Care Reporting System (NACRS), National Rehabilitation Reporting System (NRS), Ontario Cancer Registry (OCR), Ontario Drug Benefit (ODB) Program, New Drug Funding Program (NDFP), OHIP, Ontario Case Costing Initiative (OCCI), Postal Code Conversion File (PCCF), Ontario Inter-censal Population Estimates and Projections (POP), Registered Persons Database (RPDB), Standard Price (STDPRICE) and Same Day Surgery (SDS). The RPDB contains demographic information on all individuals with OHIP coverage (e.g., date of birth, date of death). The NACRS database was used to report the number of cancer clinic visits based on visit dates at any healthcare facility in Ontario. The OHIP database captures physician visits and fees for health professionals including general practitioners, medical oncologists, radiation oncologists, and other specialists. Inpatient rehabilitation admissions were captured in the NRS. Three databases were used to report treatments received by the study cohort: ODB, NDFP and ALR. ODB captures oral systemic therapy agents that are publicly funded by CCO. NDFP captures intravenous systemic therapy agents that are publicly funded by CCO. If treatment information was not available in the ODB or NDFP, treatment information from the ALR database was used.
Statistical analysis
Statistical analyses were conducted at ICES and performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA). Baseline characteristics were summarized by number and percentage for categorical variables and by mean and standard deviation (SD) for continuous variables. Staging of disease at ICES is reported using a collaborating staging methodology which combines staging from different methods to capture the highest proportion of patients (11). Charlson Comorbidity Index was also included (12). Small cells were defined as values <5, as due to privacy protocols.
Clinical outcome of interest was OS presented as mean (including SD) and median with the 95% confidence interval (CI) and interquartile range (IQR) evaluated using the Kaplan-Meier methods for censored data. OS was defined from the time of diagnosis or from initiation of treatment to death, or date of last follow-up. OS was compared in a Kaplan-Meier analysis based on stratification and log-rank test. For the survival analysis, patients were censored at death date or December, 31, 2020. Patients lost to follow-up before these dates were censored on the last day data were available for them. Statistical significance between groups was assessed using the log-rank and Chi-Square tests. Adjustment for multiple comparisons was performed using the Sidak methodology.
Results
Baseline characteristics
Our initial search of patients diagnosed with BTC in Ontario between January 1, 2010 and December 31, 2019 yielded 9,020 patients, which was reduced to 6,706 patients after applying the initial set of general exclusion criteria (Figure 1). Of these 6,706 patients only 2,142 patients (31.9%) received a treatment for BTC. Among them, 1,314 patients were diagnosed with early-stage BTC (stage I–III, unknown/missing) and became recurrent, while 828 patients were diagnosed with de novo advanced BTC (stage IV). Table 2 summarizes the patient characteristics of the cohort by stage of disease. The average age of patients in the cohort was 65.50±11.24 years. Late-stage patients were significantly older than early-stage patients, with late-stage patients having a median age of 69 years (IQR, 59–76 years) and early-stage patients having a median age of 65 years (IQR, 58–72 years) (P<0.001).
Table 2
| Baseline characteristics | BTC cohort (n=2,142) |
|---|---|
| Age at diagnosis (years) | |
| Mean ± SD | 65.50±11.24 |
| Median [IQR] | 67 [59–73] |
| Age group (years), n (%) | |
| 18–45 | 115 (5.4) |
| 46–54 | 221 (10.3) |
| 55–64 | 586 (27.4) |
| 65–74 | 746 (34.8) |
| 75+ | 474 (22.1) |
| Sex, n (%) | |
| Female | 1,066 (49.8) |
| Male | 1,076 (50.2) |
| Stage at diagnosis, n (%) | |
| Stage I | 52 (2.4) |
| Stage II | 150 (7.0) |
| Stage III | 190 (8.9) |
| Stage IV | 828 (38.7) |
| Unknown/missing stage | 922 (43.0) |
| ICD-O-3 topography code, n (%) | |
| IHCC | 702 (32.8) |
| Gallbladder | 363 (16.9) |
| EHCC | 688 (32.1) |
| AoV | 174 (8.1) |
| Malignant neoplasm of overlapping sites of biliary tract | 149 (7.0) |
| Malignant neoplasm of biliary tract, unspecified | 66 (3.1) |
| Charlson score | |
| Mean ± SD | 0.94±1.49 |
| Median [IQR] | 0 [0–1] |
| Charlson score category, n (%) | |
| 0 | 280 (13.1) |
| 1 | 102 (4.8) |
| 2 | 60 (2.8) |
| 3+ | 57 (2.7) |
| Missing | 1,643 (76.7) |
BTC, biliary tract cancer; SD, standard deviation; IQR, interquartile range; IHCC, intrahepatic cholangiocarcinoma; EHCC, extrahepatic cholangiocarcinoma; AoV, ampulla of Vater.
There were no significant differences between patients diagnosed with AoV (n=174) and non-AoV (n=1,968) for most patient characteristics such as age, income quintile and Charlson score. However, non-AoV patients were more likely to be diagnosed with stage IV cancer than AoV patients (P<0.001).
Treatment patterns
Out of the 2,142 patients in the full cohort, 1,727 patients (81%) received 1L therapy on a BTC-specific treatment (Table 3) and the majority of patients, 1,298 patients (75%), started on gem/cis. Five hundred and twelve patients (30%) went on to receive a 2L therapy, of whom 304 patients (59%) received other treatments and 109 patients (21%) received fol—while the remaining 1,215 patients received no 2L therapy. Only 44 patients (9%) went on to receive a third-line (3L) therapy.
Table 3
| Variables | Treatment type | Number of patients (%) |
|---|---|---|
| 1L | Gem/carb | 131 (7.6) |
| Gem/cis | 1,298 (75.2) | |
| Fol | 199 (11.5) | |
| Gemtaxane | 95 (5.5) | |
| Total treated in 1L | – | 1,727 |
| 2L | Gem/carb | 59 (11.5) |
| Gem/cis | 14 (2.7) | |
| Fol | 109 (21.3) | |
| Gemtaxane | 26 (5.1) | |
| Other§ | 304 (59.4) | |
| Total treated in 2L | – | 512 |
| 3L | Gem/carb | 1–5† (6.8) |
| Fol | 6 (13.6) | |
| Gemtaxane | 1–5† (6.8) | |
| Nonbtc‡ | 8 (18.2) | |
| Other§ | 27 (61.4) | |
| Total treated in 3L | – | 44 |
†, cells with a range of numbers were assumed to be the median of the range (i.e., a range of 1–5 was assumed to be 3); ‡, nonbtc regimens include any non-BTC specific regimens that are not specified in Table 1; §, other regimens include capecitabine monotherapy, capecitabine with gem, carb with a taxane, fluorouracil monotherapy, fluorouracil with gem and fluorouracil with cis. 1L, first line; 2L, second line; 3L, third line; gem, gemcitabine; carb, carboplatin; cis, cisplatin; fol, FOLFOX, FOLFIRI, FOLFIRINOX, and related regimens; Gemtaxane, gemcitabine in combination with a taxane; FOLFOX, 5-FU, FA, and oxaliplatin; FOLFIRI, 5-FU, FA, and irinotecan; FOLFIRINOX, 5-FU, FA, irinotecan, and oxaliplatin; 5-FU, 5-fluorouracil; FA, folinic acid; BTC, biliary tract cancer.
Days to treatment
Table 4 provides the average and median time on treatment for each line and the duration in between lines of therapy. Time on treatment was calculated from the day a patient received their first dose of treatment for a certain line of therapy to the day they received their last dose of that therapy line (i.e., start of 1L to last date of 1L). Time between treatments was calculated from the day after a patient received the last dose of one line of therapy to the day before they received the first dose of the next line of therapy (i.e., end of 1L+1 day to start of 2L−1 day).
Table 4
| Variables | N | Mean (SD) | Median (minimum, maximum) | IQR | |
|---|---|---|---|---|---|
| Lower quartile | Upper quartile | ||||
| Days on 1L | 1,727 | 175.2 (253.3) | 98.0 (0.0, 3,260.0) | 42.0 | 189.0 |
| Days on 2L | 512 | 101.8 (163.4) | 49.0 (0.0, 1,786.0) | 14.0 | 117.5 |
| Days on 3L | 44 | 103.0 (171.4) | 42.0 (0.0, 875.0) | 3.5 | 101.0 |
| Days from diagnosis to start of 1L | 1,726 | 182.2 (309.6) | 68.0 (0.0, 3,530.0) | 38.0 | 159.0 |
| Days from end of 1L to start of 2L | 464 | 78.9 (150.8) | 28.0 (1.0, 1,331.0) | 13.0 | 74.0 |
| Days from end of 2L to start of 3L | 38 | 94.4 (133.8) | 42.0 (8.0, 629.0) | 19.0 | 111.0 |
BTC, biliary tract cancer; SD, standard deviation; IQR, interquartile range; 1L, first line; 2L, second line; 3L, third line.
Patients spent the most time on their 1L of therapy with an average of 175.2±253.3 days and a median of 98.0 days (IQR, 42.0–189.0 days). The longest time patients spent not on treatment was from diagnosis to the start of 1L with an average of 182.2±309.6 days and a median of 68.0 days (IQR, 38.0–159.0 days).
Survival analysis
Figure 2A displays the survival curve from diagnosis to the end of follow-up or death. Median OS (mOS) for the full cohort was 11 months, while the mean survival was 20.63 months.

Figure 2B displays the survival curves from diagnosis to the end of follow-up or death, stratified by those who received 1L treatments and those who did not. As expected, patients who did not receive treatment had a significantly lower mOS than patients who received a 1L treatment (P<0.0001). Patients diagnosed with AoV had a significantly higher mOS than non-AoV patients, at 21.93 and 10.45 months, respectively (P<0.0001).
Figure 3 displays the OS from the initiation of 1L systemic therapy to the end of follow-up or death. The mOS for the full cohort from 1L was 7.4 months, with a mean of 15.7 months. Patients treated with fol or gem/cis had a mOS that was significantly higher than all treatment groups in 1L (P<0.0001); however, differences in mOS between fol and gem/cis were not significant (P=0.1433). Similar to the survival analysis of the full cohort from diagnosis comparing treated (1L) vs. untreated patients, patients diagnosed with AoV had a significantly higher mOS (10.9 months) than their non-AoV counterparts (7.2 months) (P<0.0001).
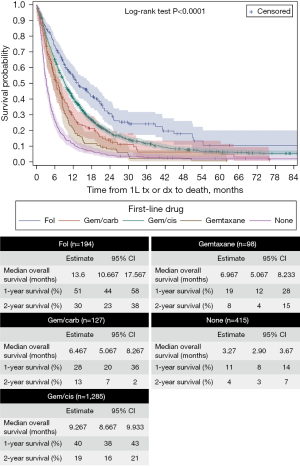
Figure 4 displays the OS from the initiation of 2L systemic therapy to the end of follow-up or death. The mOS for the full cohort from 2L was 3.9 months, with a mean of 11.5 months. The mOS for patients treated with gem/cis in 2L was not significantly higher than those treated with fol (P=0.7835) or gemtaxane (P=1.0000) but was significantly higher than that for gem/carb, other and none (P<0.05). Gem/carb was only significantly higher than none treatment group (P<0.0001). The none treatment group was significantly lower than all treatment groups in 2L (P<0.0001). Differences in mOS between AoV (9.13 months) and non-AoV (7.10 months) patients were not significant in 2L (P=0.1461).
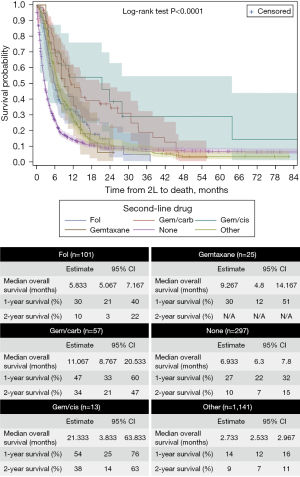
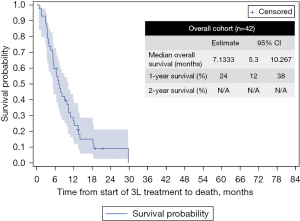
Figure 5 displays the OS from the initiation of 3L systemic therapy to the end of follow-up or death. Since a very small number of patients received 3L therapy, it was not possible to break down the survival by treatment. The mOS for the full cohort in 3L was 7.13 months, while the mean survival was 9.68 months.
Discussion
Summary
The primary objective of this study was to determine survival outcomes for patients diagnosed with BTC stratified by type of treatment received. Secondary objectives included characterizing treatment patterns associated with the management of BTC. In our study cohort of 2,142 patients, the majority were early-stage BTC (stage I–III/unknown/missing) at initial diagnosis and progressed to advanced disease, while 828 were late stage/advanced BTC (stage IV) at initial diagnosis. Most patients were diagnosed with non-AoV BTC (n=1,968). Gem/cis was the most frequently used treatment in 1L, while fol was the most commonly used among AoV patients in our cohort. These findings are consistent with current standards of care for these patients. Other treatments were the most commonly used treatment regimen in 2L, which may be due to the absence of a standard of care for 2L BTC.
The mOS for the full cohort of patients from diagnosis was 11 months. AoV patients had a significantly higher mOS (21.93 months) than non-AoV patients (10.45 months) from diagnosis. Among the full cohort, patients who received fol or gem/cis in 1L had the best mOS. After each line of therapy, there is a steep decrease in the number of patients receiving subsequent lines of therapy. Only 44 out of 1,727 patients who initiated 1L therapy received 3L therapy.
Comparisons to landmark trials and other studies
The results found in this study are slightly different from those of other Canadian studies. Beaulieu et al. [2021] conducted a population-based study of BTCs in Alberta and reported a mOS of 15 months for patients who started 1L gem/cis, whereas our study found mOS to be 9.3 months (1). However, Beaulieu et al. calculated their survival from diagnosis to death, whereas our survival calculation started from initiation of gem/cis in 1L to death (1). Similarly, Flemming et al. [2016] conducted a study looking at changes in incidence and survival of BTC in Ontario from 1994 to 2012 (2). Their study found that over time the survival for BTC has improved. Data specifically collected from 2006 to 2012 by Flemming et al. [2016] found an overall BTC mOS of 10.3 months, which is similar to the mOS found in this study (i.e., 11 months) (2). The ABC-02 study found that patients who received gem/cis in 1L had a mOS from initiation of therapy of 11.7 months (95% CI: 9.5 to 14.3 months) (13). Similarly, the TOPAZ-1 trial, which compared durvalumab plus gemcitabine and cisplatin with a placebo plus gemcitabine and cisplatin, found that patients that receive a placebo with gemcitabine plus cisplatin had a mOS of 11.5 months (95% CI: 10.1 to 12.5 months) (8). Our study found that patients who received gem/cis in 1L had a mOS of 9.3 months (95% CI: 8.67 to 9.93 months). The longer mOS reported in ABC-02 and TOPAZ-1 studies is expected since patients in a trial are a selected group compared to real-world survival outcomes where patients with co-morbidities are also allowed to receive treatment.
Another landmark trial comparing 2L FOLFOX with active symptom control, the ABC-06 trial, found that BTC patients who received 2L FOLFOX had a mOS of 6.2 months (95% CI: 5.4 to 7.6 months), which is similar to the findings of our study; namely, patients who received 2L fol had a mOS of 5.83 months (95% CI: 5.07 to 7.17 months) (14).
Limitations and bias
Our study limitations included: (I) the use of a systemic 1L treatment as a proxy for disease recurrence/progression in early-stage BTC patients. This may have introduced selection bias as early-stage patients that may have recurred but did not receive treatment or that may have received treatments other than those specified in Table 1 will not have been represented; (II) small instances of adjuvant treatments being classified as 1L therapy for BTC patients; (III) oral drug information not available for BTC patients under the age of 65 years and as well as no treatment information that was paid via private insurance or out-of-pocket; (IV) patients treated with gemcitabine monotherapy were excluded from the 1L patient population as there was no way to distinguish its use in adjuvant and metastatic setting, and we know that this regimen is commonly used in Ontario for 1L BTC. This may impact the external validity of this our study.
Future studies
Future studies should investigate the number of cycles of systemic therapy patients receive and how this affects survival. This study did not retrieve data on the exact number of cycles patients received, if patients were exposed to systemic therapy for a defined number of cycles and then had a rechallenge, or if they were treated until disease progression. Information from included databases were limited; namely, data on the breaks or rechallenges were not available with only the start and stop dates of systemic therapies recorded. Trials like the ABC-02 trial, required patients to be on certain cycles of treatment, which they used to assess survival. However, since information from included databases were unable to capture treatment cycle information, the survival analysis in this study does not determine the effect of treatment cycles on survival. Lastly, our findings highlight the importance of developing more effective systemic therapies, since only about 30% of all BTC patients received a 1L therapy and of those who received a 1L therapy, only 30% patients received further therapies. Even with innovative therapies, it is important that real-world evidence studies continue to report changes in treatment patterns of BTC patients and their impact on survival, healthcare resource utilization (HCRU) and costs.
Conclusions
This study has highlighted that the limited availability of Canadian data on BTCs has hindered the understanding of disease trends and outcomes. This study provides valuable insights into the Canadian-specific landscape of BTCs, highlighting the need for more effective treatment options. The findings underscore the importance of ongoing research and the exploration of novel therapeutic approaches to improve outcomes for patients with BTCs.
Acknowledgments
This study used de-identified data from the ICES Data Repository, which is managed by the ICES with support from its funders and partners: Canada’s Strategy for Patient-Oriented Research, the Ontario SPOR Support Unit, the Canadian Institutes of Health Research and the Government of Ontario. The opinions, results and conclusions reported are those of the authors. No endorsement by the Institute for Clinical Evaluative Sciences or any of its funders or partners is intended or should be inferred. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
Funding: This work was supported by an unrestricted research grant from AstraZeneca Canada Inc.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-155/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-155/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-155/coif). HS and SJS received support from AstraZeneca for the funding of this study and manuscript. DLC, IS and CS are employees of AstraZeneca and receive stock for AstraZeneca. EC received honoraria from AstraZeneca and participated in clinical trial sponsored by AstraZeneca. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beaulieu C, Lui A, Yusuf D, et al. A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada. Curr Oncol 2021;28:417-27. [Crossref] [PubMed]
- Flemming JA, Zhang-Salomons J, Nanji S, et al. Increased incidence but improved median overall survival for biliary tract cancers diagnosed in Ontario from 1994 through 2012: A population-based study. Cancer 2016;122:2534-43. [Crossref] [PubMed]
- Xiao Y, Cattelan L, Lagacé F, et al. Epidemiologic trends and geographic distribution of patients with gallbladder and extrahepatic biliary tract cancers in Canada. HPB (Oxford) 2021;23:1541-9. [Crossref] [PubMed]
- Cancer Care Ontario. Ch 4: Burden of Rare Cancers in Ontario [Internet]. Cancer Care Ontario. 2020 [cited 2022 Sep 29]. Available online: https://www.cancercareontario.ca/en/statistical-reports/ontario-cancer-statistics-2020/ch-4-burden-rare-cancers-ontario
- Vogel A, Bathon M, Saborowski A. Immunotherapies in clinical development for biliary tract cancer. Expert Opin Investig Drugs 2021;30:351-63. [Crossref] [PubMed]
- Canadian Cancer Society. Treatments for bile duct cancer [Internet]. Canadian Cancer Society. [cited 2022 Sep 29]. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/bile-duct/treatment/?region=on
- Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v28-37. [Crossref] [PubMed]
- Oh DY, Ruth He A, Qin S, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evidence 2022. doi:
10.1056/EVIDoa2200015 .10.1056/EVIDoa2200015 - Canadian Cancer Society. Survival statistics for gallbladder cancer [Internet]. Canadian Cancer Society. [cited 2022 Sep 29]. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/gallbladder/prognosis-and-survival/survival-statistics/?region=nb
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Cancer Care Ontario. Guidelines for Staging Patients with Cancer [Internet]. Cancer Care Ontario. [cited 2022 Sep 29]. Available online: https://ext.cancercare.on.ca/ext/databook/db1920/documents/Appendix/CCOCancerPatientStagingGuidelines.pdf.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 2021;22:690-701. [Crossref] [PubMed]



