
Prognostic factors and survival prediction in hepatocellular carcinoma: development and validation of a novel nomogram based on the SEER database
Highlight box
Key findings
• We present a novel nomogram that incorporates 9 important prognostic factors of HCC to predict CSS for HCC patients.
What is known and what is new?
• Prognostic assessment is a crucial step in the management of patients with HCC, yet there is no globally accepted staging system that is convenient enough for daily clinical practice.
• Our research adds the AFP value and BM to the construction of the nomogram, which presents impressively improved discrimination and predictive accuracy.
What is the implication, and what should change now?
• This study identifies BM as an independent variable deteriorating the survival of HCC patients, which suggests current treatment is limited for advanced HCC, especially HCC-BM. Efforts should focus on more effective therapeutic strategies for these patients.
Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and is an important medical problem. It is ranked the sixth most common neoplasm and the third leading cause of cancer deaths, with a relative 5-year survival rate of approximately 18% (1,2). Prognostic assessment is a crucial step in the management of patients with HCC. Accurate prognostic prediction in patients with HCC facilitates treatment strategy decision-making. Although different clinical staging systems of HCC have been developed in different parts of the world, such as the Cancer of the Liver Italian Program (CLIP) score, the Italian Liver Cancer (ITA.LI.CA) score, and Barcelona Clinic Liver Cancer (BCLC) staging, there is no globally accepted staging system suitable for clinical practice among heterogeneous populations (3-5). Some of these staging systems include the use of subjective components and sub-classifications, which make them not very user-friendly for daily clinical practice. Further, these systems group the patients based on treatment options and sometimes represent only a treatment decision algorithm, not prognostic evaluation (6).
A variety of prognostic factors have been evaluated and identified by previous literatures (7-9), majority of them applied to stratify patients are tumor characteristics, such as tumor size, lymph node metastasis, distant metastasis, and biomarkers related to molecular pathway. Demographic characteristics and treatment regimens also show great effect on survival, it is essential to have a prognostic model incorporating these factors to predict the outcome of individual patient of HCC with more precision. Nomogram is a graphical mathematical model which has been widely used in oncology for prognostic prediction of individual patient. It can integrate significant prognostic factors and quantify the rate of outcome to aid clinical decisions and patient-clinician communication (10).
The Surveillance, Epidemiology, and End Results (SEER) database, a publicly available cancer reporting system, has been collecting statistical information about cancers since 1975. It captures data from all clinical settings about cancer and covers approximately 28% of the U.S. population. It collects data with longitudinal follow-ups, which are related to cancer prognosis, including demographic and clinical characteristics (11). Therefore, the aim of this study was to explore the independent prognostic factors of HCC based on the SEER database and develop a novel nomogram incorporating these factors to improve predictive accuracy in patients with HCC. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-427/rc).
Methods
Data between 1975 and 2017 were evaluated using SEER*Stat software (v8.3.5; https://seer.cancer.gov/seerstat/). Patients with histologically confirmed HCC were included using the following criteria: (I) site-specific codes (C22.0) and (II) International Classification of Disease for Oncology, third edition (ICD-O 3) 8170/3 (12). The exclusion criteria were as follows: (I) patients whose information was reported from autopsy and death certificates; (II) patients who were not diagnosed as the first or only primary HCC; (III) patients with missing clinicopathological records. Of the 104,036 HCC patients recorded, 6,166 cases were finally included in the current analysis. All included cases were randomly grouped into a training cohort and a validation cohort in a ratio of 7:3. The study flowchart is shown in Figure 1.
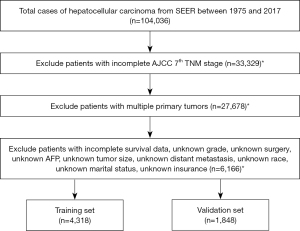
Given the public accessibility of SEER database, ethical approval was exempted and no consent was needed for this study. Baseline clinicopathologic data, including age, gender, race, insurance status, marital status, histology grade, tumor size, alpha-fetoprotein (AFP), distant metastasis, the seventh American Joint Committee on Cancer (AJCC) stage, therapy (surgery, radiation and chemotherapy) and survival time, were derived from the database. Cancer-specific survival (CSS) was defined as the interval from the initial diagnosis to the last follow-up or death due to HCC, which was set as the primary outcome. This study followed the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Baseline characteristics were compared between the training and validation cohorts by the chi-square and Fisher exact probability test. Log-rank test was used to analyze univariate factors. Multivariate Cox proportional hazard regression analysis was conducted to estimate hazard ratios and 95% confidence intervals (CIs). In the training cohort, potential predictors were assessed by univariate analysis, and those associated with survival (P<0.05) were further analyzed in multivariate analysis to identify the independent predictive factors for nomogram construction to predict the CSS rates at 2- and 5-year. To evaluate the discriminative performance of the nomogram, Harrell’s concordance index (C-index) and the area under the receiver operating characteristic (ROC) curve (AUC) were measured. The nomogram was subjected to bootstrapping validation (1,000 resamples) to construct calibration curves for comparison of the observed survival with the predicted survival in this research. Furthermore, the C-indexes of the nomogram were also compared to that of the seventh tumor-node-metastasis (TNM) staging systems. Decision curve analysis (DCA) was conducted to determine the clinical usefulness of our nomogram by quantifying the net benefits at different threshold probabilities. Statistical analyses were conducted using the software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and R version 3.4.2 (https://www.r-project.org/). The statistical significance levels were all two-sided, with statistical significance set at 0.05.
Results
Clinical characteristics
Among the 6,166 cases included in analysis, 4,318 patients were randomly assigned into the training cohort, and the remaining 1,848 patients were assigned to the validation cohort. Most were male (77.0%), white (65.3%), married (56.4%), insured (73.5%), and 60 years or older (64.1%). The most common histologic grade was grade II (47.6%), followed by grade I (29.9%), grade III (21.1%), and grade IV (1.4%). Based on the AJCC TNM stage, 43.3% of patients had stage I HCC, followed in descending order by cases with stage II (22.5%), stage III (20.9%), and stage IV (13.3%). Some 48.5% of the cases received surgery, whereas 39.2% underwent chemotherapy, and 8.6% received radiation. For regional lymph node dissection (LND), 92.5% of the cases did not have lymphadenectomy, 6.6% had 1–3 lymph nodes dissected, and 0.9% had 4 or more nodes dissected. For distant metastasis, 3.6% had spread to the lung, 2.3% to the bone, and 0.3% to the brain. In addition, most cases (67.8%) had elevated AFP levels and those remaining were normal. Among the patients with HCC, approximately half (47.0%) of the tumors were greater than 5.0 cm, 42.0% were between 2.1 and 5.0 cm in size, and 11.0% were 2.0 cm or less. Patient characteristics in the training and validation cohorts are provided in Table 1. There were no significant differences in the clinical characteristics between the training and validation cohorts.
Table 1
| Variables | Total (n=6,166) | Training cohort (n=4,318) | Validation cohort (n=1,848) | P value |
|---|---|---|---|---|
| Age, n (%) | 0.432 | |||
| <60 years | 2,214 (35.9) | 1,564 (36.2) | 650 (35.2) | |
| ≥60 years | 3,952 (64.1) | 2,754 (63.8) | 1,198 (64.8) | |
| Sex, n (%) | 0.312 | |||
| Female | 1,417 (23.0) | 977 (22.6) | 440 (23.8) | |
| Male | 4,749 (77.0) | 3,341 (77.4) | 1,408 (76.2) | |
| Race, n (%) | 0.225 | |||
| Black | 877 (14.2) | 617 (14.3) | 260 (14.1) | |
| White | 4,029 (65.3) | 2,795 (64.7) | 1,234 (66.8) | |
| Others | 1,260 (20.5) | 906 (21.0) | 354 (19.1) | |
| Marital status, n (%) | 0.990 | |||
| Married | 3,476 (56.4) | 2,434 (56.4) | 1,042 (56.4) | |
| Unmarried/single | 2,690 (43.6) | 1,884 (43.6) | 806 (43.6) | |
| Insurance, n (%) | 0.099 | |||
| Insured | 4,531 (73.5) | 3,139 (72.7) | 1,392 (75.3) | |
| Any Medicaid | 1,419 (23.0) | 1,022 (23.7) | 397 (21.5) | |
| Uninsured | 216 (3.5) | 157 (3.6) | 59 (3.2) | |
| Grade, n (%) | 0.374 | |||
| I | 1,841 (29.9) | 1,290 (29.9) | 551 (29.8) | |
| II | 2,935 (47.6) | 2,030 (47.0) | 905 (49.0) | |
| III | 1,301 (21.1) | 934 (21.6) | 367 (19.9) | |
| IV | 89 (1.4) | 64 (1.5) | 25 (1.3) | |
| AJCC TNM stage (7th), n (%) | 0.322 | |||
| I | 2,667 (43.3) | 1,864 (43.2) | 803 (43.5) | |
| II | 1,386 (22.5) | 949 (22.0) | 437 (23.6) | |
| III | 1,287 (20.9) | 910 (21.0) | 377 (20.4) | |
| IV | 826 (13.3) | 595 (13.8) | 231 (12.5) | |
| Surgery, n (%) | 0.678 | |||
| No | 3,178 (51.5) | 2,233 (51.7) | 945 (51.1) | |
| Yes | 2,988 (48.5) | 2,085 (48.3) | 903 (48.9) | |
| Dissected lymph nodes, n (%) | 0.131 | |||
| None | 5,706 (92.5) | 4,014 (93.0) | 1,692 (91.6) | |
| 1–3 | 404 (6.6) | 265 (6.1) | 139 (7.5) | |
| ≥4 | 56 (0.9) | 39 (0.9) | 17 (0.9) | |
| Chemotherapy, n (%) | 0.807 | |||
| No/unknown | 3,746 (60.8) | 2,619 (60.7) | 1,127 (61.0) | |
| Yes | 2,420 (39.2) | 1,699 (39.3) | 721 (39.0) | |
| Radiation, n (%) | 0.800 | |||
| No/unknown | 5,634 (91.4) | 3,948 (91.4) | 1,686 (91.2) | |
| Yes | 532 (8.6) | 370 (8.6) | 162 (8.8) | |
| BM, n (%) | 0.773 | |||
| No | 6,024 (97.7) | 4,217 (97.7) | 1,807 (97.8) | |
| Yes | 142 (2.3) | 101 (2.3) | 41 (2.2) | |
| Brain metastasis, n (%) | 0.510 | |||
| No | 6,150 (99.7) | 4,308 (99.8) | 1,842 (99.7) | |
| Yes | 16 (0.3) | 10 (0.2) | 6 (0.3) | |
| Lung metastasis, n (%) | 0.446 | |||
| No | 5,942 (96.4) | 4,156 (96.2) | 1,786 (96.6) | |
| Yes | 224 (3.6) | 162 (3.8) | 62 (3.4) | |
| AFP, n (%) | 0.115 | |||
| Normal | 1,987 (32.2) | 1,418 (32.8) | 569 (30.8) | |
| Elevated | 4,179 (67.8) | 2,900 (67.2) | 1,279 (69.2) | |
| Tumor size, n (%) | 0.898 | |||
| ≤2.0 cm | 676 (11.0) | 473 (11.0) | 203 (11.0) | |
| 2.1–5.0 cm | 2,587 (42.0) | 1,804 (41.8) | 783 (42.4) | |
| >5.0 cm | 2,903 (47.0) | 2,041 (47.2) | 862 (46.6) |
AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; BM, bone metastasis; AFP, alpha-fetoprotein.
Predictor selection and nomogram construction
Multiple demographic and clinical features were analyzed for identification of the potential prognostic indicators (Table 2). The univariate analysis indicated that race, histological grade, AJCC TNM stage, surgery, chemotherapy, radiation, bone metastasis (BM), AFP, and tumor size were statistically significant factors for CSS in the training cohort. Further multivariate Cox analyses identified the above factors as independent prognostic predictors for CSS, and they were incorporated into the prognostic nomogram.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Log-rank χ2 | P value | HR (95% CI) | P value | ||
| Sex | 11.543 | 0.001 | |||
| Female | Reference | ||||
| Male | 1.051 (0.945–1.169) | 0.357 | |||
| Age | 14.145 | 0.000 | |||
| <60 years | Reference | ||||
| ≥60 years | 1.042 (0.952–1.140) | 0.370 | |||
| Race | 44.516 | 0.000 | 0.001 | ||
| Black | Reference | ||||
| White | 1.010 (0.898–1.136) | 0.864 | |||
| Others | 0.812 (0.699–0.943) | 0.006 | |||
| Marital status | 28.712 | 0.000 | |||
| Married | Reference | ||||
| Unmarried/single | 1.026 (0.938–1.123) | 0.569 | |||
| Insurance | 27.873 | 0.000 | 0.967 | ||
| Insured | Reference | ||||
| Any Medicaid | 0.998 (0.901–1.105) | 0.968 | |||
| Uninsured | 1.027 (0.831–1.269) | 0.806 | |||
| Grade | 201.366 | 0.000 | 0.000 | ||
| I | Reference | ||||
| II | 1.136 (1.024–1.260) | 0.016 | |||
| III | 1.760 (1.564–1.981) | 0.000 | |||
| IV | 2.032 (1.504–2.746) | 0.000 | |||
| AJCC TNM stage (7th) | 1403.765 | 0.000 | 0.000 | ||
| I | Reference | ||||
| II | 1.274 (1.119–1.450) | 0.000 | |||
| III | 1.759 (1.555–1.989) | 0.000 | |||
| IV | 2.649 (2.286–3.070) | 0.000 | |||
| Surgery | 1406.046 | 0.000 | |||
| No | Reference | ||||
| Yes | 0.209 (0.186–0.236) | 0.000 | |||
| Dissected lymph nodes | 83.497 | 0.000 | 0.159 | ||
| None | Reference | ||||
| 1–3 | 0.775 (0.598–1.006) | 0.055 | |||
| ≥4 | 0.997 (0.575–1.729) | 0.991 | |||
| Chemotherapy | 27.702 | 0.000 | |||
| No/unknown | Reference | ||||
| Yes | 0.544 (0.496–0.596) | 0.000 | |||
| Radiation | 31.785 | 0.000 | |||
| No/unknown | Reference | ||||
| Yes | 0.644 (0.559–0.742) | 0.000 | |||
| BM | 181.423 | 0.000 | |||
| No | Reference | ||||
| Yes | 1.482 (1.165–1.884) | 0.001 | |||
| Brain metastasis | 17.923 | 0.000 | |||
| No | Reference | ||||
| Yes | 0.823 (0.415–1.630) | 0.576 | |||
| Lung metastasis | 309.406 | 0.000 | |||
| No | Reference | ||||
| Yes | 1.137 (0.933–1.388) | 0.204 | |||
| AFP | 145.282 | 0.000 | |||
| Normal | Reference | ||||
| Elevated | 1.391 (1.259–1.536) | 0.000 | |||
| Tumor size | 671.436 | 0.000 | 0.000 | ||
| ≤2.0 cm | Reference | ||||
| 2.1–5.0 cm | 1.623 (1.326–1.986) | 0.000 | |||
| >5.0 cm | 2.509 (2.038–3.089) | 0.000 | |||
CSS, cancer-specific survival; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; BM, bone metastasis; AFP, alpha-fetoprotein.
Performance and validation of the novel nomogram
Based on the independent prognostic factors, a novel nomogram was established for predicting the 2- and 5-year CSS of the patients (Figure 2). The C-index for the prediction nomogram was 0.802 (95% CI, 0.792–0.812) for the training cohort, and 0.801(95% CI, 0.787–0.815) for the validation cohort. The AJCC TNM staging system yielded a C-index of 0.686 (95% CI, 0.674–0.698) for the training set and 0.693 (95% CI, 0.676–0.711) for the validation set. The nomogram’s discrimination ability was significantly superior to that of AJCC stage (P<0.001). The calibration curve of the novel nomogram for prediction of 2- and 5-year CSS demonstrated good agreement between prediction and observation both in the training and validation groups (Figure 3). The AUCs of the nomogram are displayed in Figure 4. The AUCs of the 2- and 5-year CSS nomogram in the training cohort were 0.867 and 0.842, respectively, whereas those of the seventh TNM staging system were 0.739 and 0.706, respectively. Improved prognostic prediction of 2- and 5-year CSS were observed for the nomogram. Similar findings were also demonstrated in the verification cohort (Table 3).
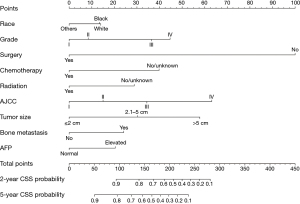
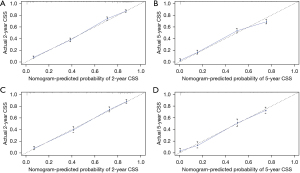
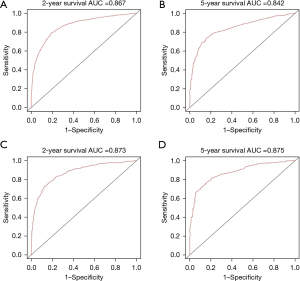
Table 3
| Metrics | Training cohort | Validation cohort | |||||
|---|---|---|---|---|---|---|---|
| Nomogram | AJCC stage | P value | Nomogram | AJCC stage | P value | ||
| C-index (95% CI) | 0.802 (0.792–0.812) | 0.686 (0.674–0.698) | <0.001 | 0.801 (0.787–0.815) | 0.693 (0.676–0.711) | <0.001 | |
| AUC | |||||||
| 2-year CSS | 0.867 | 0.739 | 0.873 | 0.735 | |||
| 5-year CSS | 0.842 | 0.706 | 0.875 | 0.717 | |||
AJCC, American Joint Committee on Cancer; HCC, hepatocellular carcinoma; CI, confidence interval; AUC, area under the ROC curve; ROC, receiver operating characteristic; CSS, cancer-specific survival.
Clinical use
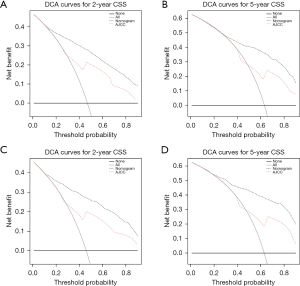
The DCA for the novel nomogram and TNM staging system is presented in Figure 5. Compared with the TNM stage, the nomogram showed more net benefits with a wide range of threshold probabilities at 2- and 5-year CSS in both the training and validation cohorts, which indicated the prediction model would bring more favorable effect in the clinical decision-making.
Discussion
We explored the independent prognostic factors of CSS in patients with HCC based on the SEER database and integrated them into an easy-to-use nomogram for the individualized prediction of 2- and 5-year CSS. The novel nomogram incorporates 9 items including race, histological grade, surgery, chemotherapy, radiation, AJCC stage, tumor size, BM, and AFP. The nomogram successfully assisted the prediction of the 2- and 5-year CSS in patients with HCC and demonstrated adequate clinical practicability.
For the construction of the novel nomogram, 16 candidate predictors were evaluated for their prognostic association with CSS. By univariate and multivariate analyses, 9 factors were found to be independently relative to prognosis. Survival analyses confirmed previous findings of prognostic significance of race, histological grade, TNM stage, and surgery (9,13,14). Serum AFP was not captured by SEER until 2004 and was therefore not included in previous studies (9,13). Increasing AFP values are associated with poor prognosis and higher tumor recurrence rate in patients with HCC (15). In this regard, we included the AFP factor and observed a worse prognosis in both the training and validation cohorts independently.
Controversial evidence exists regarding the independent significance of age and tumor size in predicting HCC survival (13,16-18). Our study found no significant difference existed in CSS between younger (<60 years) and elderly patients (≥60 years). One possible explanation for this might be the progressively improving effectiveness of treatment for HCC, including palliative locoregional treatment and tyrosine-kinase inhibitors. De Toni et al. found an age independent survival benefit for patients with HCC without metastases at diagnosis (19). Cucchetti et al. also suggested that surgery in elderly patients can achieve the greatest benefit in terms of lifespan from birth (20). Some previous studies have not integrated tumor size into the prognostic prediction model (13,21), its controversial predictive value may be due to the cut-offs chosen. According to the eighth AJCC staging system (22), we applied the tumor size cut-offs of ≤2.0 cm, 2.1–5.0 cm, and >5.0 cm, which demonstrated a significantly worse survival in patients with larger tumor size. This observation supported incorporating tumor size as an independent stratification factor into our scoring model.
In the past decade, treatment of HCC has evolved considerably (1,23,24). Patients with HCC can benefit from appropriate treatment approaches that improve their survival whatever the tumor stage at diagnosis. Surgical resection, transplantation, ablation, intra-arterial therapies, radiotherapy, and systemic therapies have all been shown to have survival benefit for appropriate candidates (25,26). Our analysis of surgery, radiotherapy, and chemotherapy as the treatment variables of CSS confirmed they all could be beneficial to patients’ outcome, and surgery was the best strategy for improving survival. Another potentially relevant issue was the value of LND in HCC. Some researchers have suggested that routine LND had benefits in outcome improvement, complication prevention, and comprehensive evaluation (8,27), but others hold opposite opinions (28,29). We investigated the relationship of LND with the CSS in patients with HCC and found no independent correlation with the CSS, therefore, lymph nodes dissected were not considered for incorporation into the predictive model. The AJCC staging system has been widely accepted and universally adopted by clinicians for assessment of tumor stage. Therefore, the TNM stage was integrated to the nomogram for prognosis prediction.
Although implementation of screening programmes and the advances in the therapeutic strategies have contributed to improve survival of HCC patients (9,30,31), treatment options for patients with advanced disease are still very limited and prognosis of these patients remains dismal (32-34). Extrahepatic metastasis of HCC may occur at initial diagnosis or during recurrence following treatment. We examined the extrahepatic metastasis-association with the survival of patients and observed that patients with distant spreads had expectedly poorer prognoses. The most common site of metastasis of HCC was the lung, followed by the bone and brain, which was consistent with the previous studies (33,35). Furthermore, we evaluated the involved organ with the 2- and 5-year CSS and found that BM was independently associated with worse survival compared to metastasis to other organs. The vertebrae have been reported to be the most frequent site of osseous metastasis (35), portal hypertension might account for the predilection (36), which suggests that HCC-BM patients may have a higher rate of decompensated cirrhosis and increased risk of mortality. However, this hypothesis needs further investigation. An effective treatment has not yet been established for HCC-BM, and bone pain and/or neurological deficits are the frequent symptoms affecting patients’ quality of life. External bone radiation has been used as the palliative therapy, but has failed to demonstrate survival benefit (37,38). More effective therapeutic interventions are urgently required to improve the quality of life and extend the lives of patients with extrahepatic spread, especially with BM (39). Recently, many clinical trials involving immune checkpoint inhibitors have been conducted for advanced HCC, exploring novel avenues to improve therapeutic efficacy (40). The impressive observation of independent BM-survival association led us to combine this predictor to the construction of the nomogram.
We presented and validated a novel nomogram in this study, which integrated demographic and clinical variables for survival prediction of HCC. This nomogram provided satisfactory discrimination (C-index 0.802, in the primary cohort, and C-index 0.801, in validation cohort), and showed improved predictive accuracy (AUCs 0.873 for 2-year CSS and 0.875 for 5-year CSS respectively, in the validation cohort) compared with other prognostic models previously reported (5,13,21,41). Even though this easy-to-use scoring system presented good discrimination and calibration, we still need to justify the clinical usefulness. With this aim, DCA was applied in our study, which showed that the novel nomogram for HCC survival prognosis yielded more benefit than either the AJCC staging system or the treat-all-patients scheme.
The current study had both strengths and limitations. Compared with previous reports, we combined the AFP values and BM to the construction of the nomogram and applied it for prognostic evaluation of the general population of all HCC stages with impressively improved discrimination and predictive accuracy. However, this study is limited by its retrospective nature and that the SEER database is mainly based on the American population, the applicability of the nomogram in other geographic populations such as Asia still needs to be confirmed. Additionally, the SEER database does not allow for the assessment of the hepatic function reserve, which is important for the prognostication for HCC patients, especially for those with a background of chronic liver disease. Its scope of applicability in clinical practice should be investigated further by multi-institutional prospective validation.
Conclusions
In conclusion, this study presents an encouraging nomogram based on the variables that are independently associated with the survival in HCC patients to better discriminate the prognosis, which will facilitate individualized management of HCC.
Acknowledgments
Funding: This work was supported by the Zhejiang Medical and Health Plan (No. 2023KY910) and Wenzhou Science and Technology Project (No. Y20210920).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-427/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-427/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-427/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345-62. [Crossref] [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 2000;32:679-80. [Crossref] [PubMed]
- Farinati F, Vitale A, Spolverato G, et al. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med 2016;13:e1002006. [Crossref] [PubMed]
- Tellapuri S, Sutphin PD, Beg MS, et al. Staging systems of hepatocellular carcinoma: A review. Indian J Gastroenterol 2018;37:481-91. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Bergquist JR, Li AY, Javadi CS, et al. Regional lymph node sampling in hepatoma resection: insight into prognosis. HPB (Oxford) 2021;23:1360-70. [Crossref] [PubMed]
- Ding J, Wen Z. Survival improvement and prognosis for hepatocellular carcinoma: analysis of the SEER database. BMC Cancer 2021;21:1157. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Park HS, Lloyd S, Decker RH, et al. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer 2012;36:183-90. [Crossref] [PubMed]
- Fritz A, Percy C, Jach A, et al. editors. International classification of diseases for oncology: ICD-O. 3rd ed. Geneva: World Health Organization, 2000.
- He T, Chen T, Liu X, et al. A Web-Based Prediction Model for Cancer-Specific Survival of Elderly Patients With Early Hepatocellular Carcinoma: A Study Based on SEER Database. Front Public Health 2022;9:789026. [Crossref] [PubMed]
- Brar G, Greten TF, Graubard BI, et al. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatol Commun 2020;4:1541-51. [Crossref] [PubMed]
- Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020;9:1370. [Crossref] [PubMed]
- Yan B, Su BB, Bai DS, et al. A practical nomogram and risk stratification system predicting the cancer-specific survival for patients with early hepatocellular carcinoma. Cancer Med 2021;10:496-506. [Crossref] [PubMed]
- Kluger MD, Salceda JA, Laurent A, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol 2015;62:1131-40. [Crossref] [PubMed]
- Kim J, Ko ME, Nelson RA, et al. Increasing age and survival after orthotopic liver transplantation for patients with hepatocellular cancer. J Am Coll Surg 2014;218:431-8. [Crossref] [PubMed]
- De Toni EN, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut 2020;69:168-76. [Crossref] [PubMed]
- Cucchetti A, Sposito C, Pinna AD, et al. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br J Surg 2016;103:e93-9. [Crossref] [PubMed]
- Xiao Z, Yan Y, Zhou Q, et al. Development and external validation of prognostic nomograms in hepatocellular carcinoma patients: a population based study. Cancer Manag Res 2019;11:2691-708. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol 2020;72:262-76. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Vogel A, Martinelli EESMO Guidelines Committee, et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-5. [Crossref] [PubMed]
- Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 2021;75:960-74. [Crossref] [PubMed]
- Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg 2004;239:202-9. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:541-65. [Crossref] [PubMed]
- Amini N, Ejaz A, Spolverato G, et al. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg 2014;18:2136-48. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;77:128-39. [Crossref] [PubMed]
- Si MS, Amersi F, Golish SR, et al. Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival. Am Surg 2003;69:879-85. [Crossref] [PubMed]
- Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 2005;20:1781-7. [Crossref] [PubMed]
- Elmoghazy W, Ahmed K, Vijay A, et al. Hepatocellular carcinoma in a rapidly growing community: Epidemiology, clinico-pathology and predictors of extrahepatic metastasis. Arab J Gastroenterol 2019;20:38-43. [Crossref] [PubMed]
- Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int 2008;28:1256-63. [Crossref] [PubMed]
- Yuan X, Zhuang M, Zhu X, et al. Emerging Perspectives of Bone Metastasis in Hepatocellular Carcinoma. Front Oncol 2022;12:943866. [Crossref] [PubMed]
- Bhatia R, Ravulapati S, Befeler A, et al. Hepatocellular Carcinoma with Bone Metastases: Incidence, Prognostic Significance, and Management-Single-Center Experience. J Gastrointest Cancer 2017;48:321-5. [Crossref] [PubMed]
- Jung IH, Yoon SM, Kwak J, et al. High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget 2017;8:15182-92. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019;70:817. [Crossref] [PubMed]
- Ouyang T, Kan X, Zheng C. Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: Monotherapies and Combined Therapies. Front Oncol 2022;12:898964. [Crossref] [PubMed]
- Liu K, Huang G, Chang P, et al. Construction and validation of a nomogram for predicting cancer-specific survival in hepatocellular carcinoma patients. Sci Rep 2020;10:21376. [Crossref] [PubMed]
(English Language Editor: J. Jones)


