
Current management of gastric adenocarcinoma: a narrative review
Introduction
Stomach cancer was the fifth most common cancer worldwide in 2020 (excluding non-melanoma skin cancer) with 1.1 million new cases, and the fourth leading cause of cancer death with approximately 800,000 deaths (1,2). Incidence rates are highest in Eastern Asia and Eastern Europe, while rates in Northern America and Northern Europe are equivalent to those in Africa and are generally low (1,2). Incidence and mortality rates from non-cardia gastric cancer (GC) are declining in the last half a century while the relative increase seen in gastric cardia cancers appears to be stabilized, at least for the United States and Netherlands (1-6). A new and contrasting observation, that warrants further validation, is an incidence increase in GC (both cardia and noncardia) in young adults aged 50 and below in both high and low-risk countries, and particularly in males (7,8).
Clinical staging dictates prognosis and treatment strategy. Using the National Cancer Database, the 5-year survival estimates in the 8th edition of the American Joint Committee on Cancer (AJCC) manual were IA: 81%; IB: 68.5%; IIA: 59.3%; IIB: 46.4%; IIIA: 30.5%; IIIB: 20.1%; IIIC: 8.3%; IV: 5.6% (9). According to the Surveillance, Epidemiology, and End Results Program (SEER), the stage distribution of cases in 2011–2019 was 29.4% for localized disease, 24.4% for regional disease, 33.6% for distant advanced disease and 12.6% unknown (10). The high incidence of GC in the eastern countries has led to the implementation of population-based screening programs. Those programs may result in a ‘stage-shift’ with earlier-stage detection where more curative intent treatments are available (11).
Rational and objective
From organ sparing endoscopic resection for early disease, through multimodality treatments with minimally invasive surgical approaches and novel targeted therapies for metastatic disease, the management of gastric adenocarcinoma has advanced considerably over the past few years. This review will discuss the current management gastric adenocarcinoma and recent therapeutic advances.
Gastroesophageal junction (GEJ) adenocarcinoma
For this review on gastric adenocarcinoma, we will apply only on Siewert type III GEJ (12).
Siewert type I tumors that arise from the distal esophagus and infiltrate the GEJ from above and tumors of the true GEJ (Siewert type II) will not be discussed in this review.
Methods
This review is based on literature search that was conducted in June 2022 through PubMed database and ClinicalTrials.gov registry. We included phase 2/3 studies as well as retrospective and observational analysis (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | June 2022 |
| Databases and other sources searched | PubMed, ClinicalTrials.gov |
| Search terms used | Gastric cancer, endoscopic resection, EMR, ESD, perioperative, neoadjuvant, adjuvant, metastatic, targeted therapy, immune checkpoint inhibitors |
| Timeframe | Up to June 2022 |
| Inclusion and exclusion criteria | Excluded: non-English studies |
| Selection process | Y Nevo conducted the selection; L Ferri approved the selection of studies |
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.
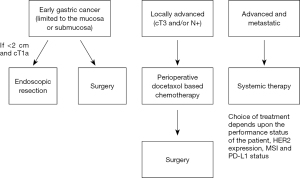
Early gastric cancer (EGC), localized disease
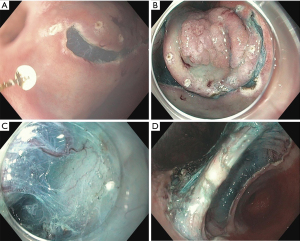
EGC is defined as adenocarcinoma limited to the mucosa or submucosa (Figure 1), regardless of lymph node metastasis (11,13). The mainstay of treatment is resection, either endoscopic resection or surgery. Endoscopic resection, either endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) is widely adopted in the East and has shown successful results in the treatment of EGC (14-16). Endoscopic resection is advised for tumors that have a very low possibility of lymph node metastasis and are amendable for complete resection (17). A multicenter retrospective study from Korea reporting EMR long-term outcomes in EGC found no cancer-related death in more than 3 years of follow-up and only 6% local recurrence rate (18). EMR is a valid option in patients with EGC, but proper patient selection is important to achieve good clinical outcomes. Achieving complete resection by EMR is dependent upon several parameters including the gross endoscopic type, the tumor grade, and the depth of invasion (14). As stated by the European Society of Gastrointestinal Endoscopy, EMR is a valid option for lesions smaller than 10–15 mm with a very low probability of advanced histology (19). EMR has some limitations and often results in piecemeal resection that may lead to high local recurrence rates (20). ESD allows for en-bloc resection (Figure 2) and precise histological assessment of EGC and has been shown to be more effective than EMR (20-23). For lesions greater than 5 mm the complete resection rate was significantly better for ESD than EMR, whereas for lesions less than 5 mm the rates were not different (20,23,24). ESD has also shown comparable outcomes to surgery for EGC in terms of overall and disease specific survival. it is usually associated with shorter hospital stay, lower cost, and better quality of life (25-27). ESD has become the treatment of choice for EGC in Asia (28,29) and is gaining acceptance in the West (30). According to National Comprehensive Cancer Network (NCCN) guideline (version 2.2022) EGC that is less than or equal to 2 cm in diameter, well to moderately differentiated, does not invade submucosa, does not exhibit lymph-vascular invasion (LVI) and has clear lateral and deep margins can be treated with EMR or ESD (12). The Japanese Gastric Cancer association (JGCA) guidelines (5th edition) further include two more types of EGC as an indication for ESD: a differentiated-type adenocarcinoma without ulcerative findings, in which the depth of invasion is clinically diagnosed as T1a and the diameter is >2 cm. A differentiated-type adenocarcinoma with ulcerative findings, in which the depth of invasion is clinically diagnosed as T1a and the diameter is ≤3 cm (31). For EGC tumors that do not fulfil those indications surgical resection should be considered.
Locally advanced GC
The treatment strategy for locally advanced GC (Figure 3) has changed from surgery alone to a multimodality approach. However, surgery alone offers poor overall survival (OS) whereas the multimodality therapy has been shown to significantly increase OS in patients with locally advanced disease (32-35).
Surgical resection
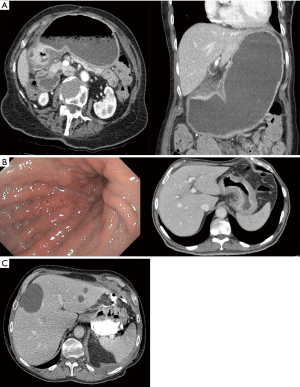
The principle of surgery includes gastrectomy with clear margins and appropriate lymph node dissection. Currently, there is no consensus as for the adequate distance between the tumor and the resection margin to ensure complete tumor excision with negative margins (R0) and minimize the risk for local recurrence. While previously recommending resection margins >4 cm from the gross tumor, the recent (version 2.2022) NCCN guideline recommends adequate gastric resection to achieve negative microscopic margins (12). In the European Society of Medical Oncology (ESMO) recommends a 5-cm macroscopic proximal margin for intestinal type cancers and 8 cm in diffuse type cancers when performing a subtotal gastrectomy. If those margins cannot be secured, a total gastrectomy is advised (36). In the JGCA guidelines the growth pattern is considered. In an expensive growth pattern for a proximal margin of 3 cm is recommended whereas for an infiltrative growth pattern a 5-cm margin is advised. A frozen section is recommended when those margins cannot be secured (31,37). The safety and efficacy of the laparoscopic approach has been evaluated in several trials. The KLASS-02-RCT was a Korean trial which showed lower rate of complications in laparoscopic gastrectomy with D2 lymphadenectomy for locally advanced GC (16.6% vs. 24.1%, P=0.003), less post-operative pain, and shorter length of hospital stay (8.1 vs. 9.3 days, P=0.005) compared with open surgery (38). The long-term outcomes of the KLASS-02 trial demonstrated comparable disease-free survival (DFS) outcomes (39). The LOGICA trial was a multicenter Western RCT comparing laparoscopic to open gastrectomy that did not find a difference in complication rate (44% vs. 42%, P=0.91) or length of stay (7 days in both groups, P=0.34), though the cohort included also total gastrectomy. The oncological efficacy including R0 resection rate (95% vs. 95%, P=1.00), and median lymph node yield (29 vs. 29 nodes, P=0.49) was similar in both groups (40). Accordingly, we recommend performing gastrectomy in the approach, laparoscopic or open, for which local expertise exists as both approaches are acceptable and can achieve the stated goals of the operation, safe resection of the tumour in an ontologically appropriate manner (negative margins and extended lymphadenectomy). In addition, there are some conditions which may not lend themselves to a laparoscopic approach for which an open operation may be better suited including gastric outlet obstruction with a massively dilated stomach (Figure 4A), very bulky gastric tumours with linitis plastica (Figure 4B), and perforated tumours (Figure 4C).
Lymph node dissection
The extent of lymph nodes dissection is still a matter of ongoing debate. D1 lymphadenectomy includes removal of all the perigastric lymph nodes, whereas a D2 lymph nodes dissection involves the removal of nodes along the vessels including the left gastric, common hepatic, celiac, and splenic artery (Figure 5). In the JGCA guidelines, D2 dissection is indicated for potentially curable T2–T4 tumours, as well as for cT1N+ tumors (11,31). Six randomized controlled trials (RCTs) compared a more limited to extended lymphadenectomy in GC (41-47). Initial results from two large RCTs performed at Netherlands and UK didn’t find a significant survival benefit of D2 vs. D1 lymph nodes dissection (43,44). In a 15-year median follow-up of the Dutch trial, it was shown that D2 lymphadenectomy significantly reduced recurrence and death rates (48). The Taiwanese RCT demonstrated a benefit of extended lymphadenectomy on 5-year OS and 5-year disease-specific survival (45) and in all other RCTs survival rates were similar between D1 and D2 lymphadenectomy. The Italian trial showed that D1 lymphadenectomy may be better patients older than 70 years old and in early GC (46). Both the Dutch the British and other trials showed increased morbidity and mortality after D2 dissection largely attributed to splenectomy and/or pancreatectomy, once a mandatory part of the D2 lymphadenectomy (43,44,49). After adjustment for splenectomy and/or pancreatectomy, the morbidity difference that was seen in the British trial became non-significant. In the Dutch trial splenectomy or pancreatico-splenectomy decreased mean OS after 15 years in both the D1 and D2 dissection group. A significant 15-year survival benefit was seen when D2 lymphadenectomy was done without splenectomy and/or pancreatectomy. Demonstrated also by the Taiwanese RCT with increased 5-year OS and DFS with spleen and/or pancreas preserving lymphadenectomy (43-45,47). A meta-analysis including the Dutch, the British and the Taiwanese RCTs concerning lymphadenectomy with or without pancreatico-splenectomy demonstrated a survival benefit for D2 compared to D1 (50). D2 lymphadenectomy improves survival as long as the morbidity is kept low, without splenectomy/pancreatectomy unless properly indicated.
Multimodality treatment
The multimodality approach has been proven to prolong survival in locally-advanced gastric adenocarcinoma (34,35). In Western countries, perioperative chemotherapy with a docetaxel-based triplet therapy {e.g., FLOT [5-fluorouracil (5-FU), leucovorin, oxaliplatin, and docetaxel]} before and after surgery is the standard. In Asian countries, like Japan, Korea and China, adjuvant chemotherapy is preferred (11). Chemotherapy in the pre-operative setting is usually better tolerated than in the post-operative period, and may improve survival due to eradication of occult micrometastasis (51); however, both approaches are well established and accepted.
Perioperative chemotherapy
The perioperative approach was introduced by the MAGIC trial, in which 503 patients with stage II or greater adenocarcinoma of the stomach, GEJ and lower oesophagus were randomized to receive ECF (epirubicin, cisplatin, and 5-FU) before and after surgery or surgery alone (52). Perioperative ECF demonstrated a significant improvement in 5-year OS (36% vs. 23%, P=0.009) and progression-free survival (PFS) (35% vs. 25%, P<0.001). A subsequent study, the French ACCORD-07 trial (53) demonstrated similar results and survival benefit of perioperative chemotherapy and surgery over surgery alone (5-year OS: 38% vs. 24%, P=0.02) though using doublet regimen of fluoropyrimidine/platinum instead of an anthracycline-based triplet regimen of the MAGIC trial. The FLOT4 study randomized patients to receive perioperative FLOT or ECF.
DFS and OS were significantly improved in patients who received FLOT (DFS: 30 vs. 18 months, P=0.0036; OS 50 vs. 35 months, P=0.012). Postoperative complications and mortality were similar in both groups (54). For medically fit patients, FLOT is now considered standard of care perioperative chemotherapy in gastric adenocarcinoma. However, not all eligible patients with locally-advanced GC are amenable to receive neoadjuvant therapy due to clinical scenarios precluding their ability to tolerate the treatment. These include the significant bleeding, gastric outlet obstruction, or perforation with active infection. In such cases it is recommended for the patient to undergo upfront resection, if feasible, followed by some forms of adjuvant therapies (chemotherapy or chemoradiation—see below).
Adjuvant chemotherapy
Surgical resection followed by adjuvant chemotherapy is the standard of care in Asia, and most of the data supporting this approach comes from Asian countries (55,56). The Japanese Adjuvant Chemotherapy Trial of S-1 for GC (ACTS-GC) trial examined the role of adjuvant S-1 (oral fluoropyrimidine) following surgery with D2 lymphadenectomy for stage II and III gastric adenocarcinoma (55). The adjuvant chemotherapy group of S-1 demonstrated superior 5-year OS outcomes compared with surgery alone [71.7% vs. 61.1%, 95% confidence interval (CI): 0.54–0.83] (57).
The CLASSIC study was a phase III RCT which randomized 1,035 patients with stage II–IIIB GC who underwent gastrectomy D2 lymph node dissection to receive adjuvant chemotherapy (capecitabine and oxaliplatin) or observation only. Five-year OS was significantly improved in the chemotherapy group (78% vs. 69%, P=0.0015) (56,58). Adding taxane in the adjuvant setting has shown improved outcomes in the Japanese Clinical Cancer Research Organization (JACCRO) GC-07 trial, in which 915 patients with lymph node-positive stage IIA–IVA GC were randomized following curative gastrectomy with D2 lymphadenectomy to postoperative S-1 plus docetaxel or S-1. The docetaxel group had improved 3-year relapse-free survival (RFS) (66% vs. 50%, P<0.001) (59).
Adjuvant chemoradiotherapy
Since the introduction of the landmark Intergroup 0116 trial (INT-0116) (32), more than 20 years ago, post-operative chemoradiotherapy is no longer the standard of care for resectable gastric adenocarcinoma, and is instead reserved for patients with residual disease following surgery or those who received less than D2 lymphadenectomy (12,51). In the INT-0116, 556 patients were randomized to receive postoperative chemotherapy and chemoradiation with 5-FU/leucovorin or surgery followed by observation alone, and both showed improved 3-year OS (50% vs. 41%, P=0.005) and 3-year RFS (48% vs. 31%, P<0.001) (32), with continued strong benefit from the tri-modality treatment at 10-year follow-up (60). A reduction in local and regional recurrence was primarily responsible for the survival benefit in the chemoradiotherapy group (19% vs. 29% and 65% vs. 72%, respectively). However, the study was criticized as only 10% of patients underwent formal D2 lymphadenectomy and 54% underwent D0 dissection. A subsequent phase III trial, the Korean ARTIST trial examined the effects of radiation following surgery with appropriate lymph node dissection, but showed no benefit in survival (61). Patients were randomized to receive adjuvant chemotherapy (capecitabine and cisplatin) alone or in combination with radiotherapy. Five-year OS (75% vs. 73%) and 3-year DFS (78% vs. 74%) were similar in the two groups (61,62). The adjuvant chemoradiotherapy treatment of patients with node-positive cancer demonstrated improved DFS, but a subsequent study (ARTIST-II) specifically designed to evaluate this subgroup of patients who had undergone D2 lymphadenectomy and were node-positive did not show any significant difference in DFS when combined with adjuvant chemoradiotherapy. (63). The Dutch CRITICS trial also did not show any significant difference in the median OS of perioperative ECF or preoperative ECF followed by adjuvant chemoradiation (37 vs. 43 months, P=0.90) (64). Taken together these data suggest that radiation therapy may not provide benefit patients for whom adequate local control was achieved with surgery. However, in patients with pathologically proven locally advanced disease (pT3 and/or N1-3) who did not receive a formal D2 dissection or with positive margins, the addition of radiation to chemotherapy in the adjuvant setting is recommended despite the absence of solid phase 3 data (65). Figure 6 illustrates our current approach to managing gastric adenocarcinoma.
Perioperative targeted therapies
Targeted therapies in GC include monoclonal antibodies directed against Vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor-2 (VEGFR-2), and human epidermal growth factor receptor 2 (HER2). In patients with advanced/metastatic GC, targeted therapies have become the standard of care in addition to chemotherapy (66-68), and was studies as a possible combination in the context of perioperative care. As a part of the UK ST03 study, bevacizumab an anti-VEGFR monoclonal antibody was added to preoperative ECX chemotherapy, but no survival benefits were observed. Additionally, bevacizumab was associated with increased wound healing complications (69). According to the phase II FLOT7-RAMSES trial reported in an abstract form, ramucirumab was added to perioperative FLOT to improve R0 resection rates (97% vs. 83%, P=0.0049), but not the pathological response (27% vs. 30%, P=0.7363). The Ramucirumab arm had increased mortality (5.9% vs. 2.5%), that occurred in patients with Siewert type I GEJ adenocarcinoma (70). Approximately 10-25% of GC’s overexpress HER2. The PETRARCA phase II randomized trial reported as an abstract, showed that the addition of trastuzumab/pertuzumab (anti HER2 monoclonal antibodies) to perioperative FLOT significantly improves pathological complete response (pCR) rate (35% vs. 12%, P=0.02) on the other hand, the extent of diarrhea and leukopenia has increased (71). In the Dutch phase II HER-FLOT single-arm trial, trastuzumab was combined with FLOT in 56 patients with locally advanced resectable gastro-esophageal adenocarcinoma (72). The primary endpoint of pCR >20% was reached, with approximately 50% of patients achieving complete or near-complete remission and the 3-year OS rate was 82.1%. There were only mild adverse reactions. Further results are needed to demonstrate the effectiveness of HER2 targeted agents in the perioperative treatment of HER2-positive GC (73).
Biomarkers
Molecular markers and histopathologic tumor features are recently appearing as important factors in patients’ outcomes (74-76). Cancer Genome Atlas (TCGA) and Asian Cancer Research Group (ACRG) have developed detailed genomic characterizations of GC (74,75). The TGCA identified four molecular subtypes of OG cancer—Epstein-Barr virus (EBV)-positive, microsatellite instability (MSI), genomic stable and chromosomal unstable tumour subtypes (74)—and the ACRG identified MSI, microsatellite stable/epithelial to mesenchymal transition (MSS/EMT), MSS/TP53+ and MSS/TP53− as subtypes with prognostic values (75).
MSI
In patients with GC, MSI varies greatly (74,77,78) based on the ethnic origin of the cohorts (Asian vs. Caucasian), tumor heterogeneity, and MSI assays used. There is a significant difference between MSI for node-negative disease, which is up to 20%, and MSI for metastatic disease, which is only 5% (75,78). In addition to an older age (65 years) and female gender, MSI GCs are less likely to involve lymph nodes, and to invade serosal layers (78-80). There was a greater survival benefit in patients with MSI high compared to microsatellite stable (MSS) patients in a meta-analysis of the MAGIC, CLASSIC, ARTIST, and ITACA-S trials (77.5% vs. 59.3%). However, those with MSI-high who received chemotherapy and surgery had worse outcomes than patients who underwent surgery alone (5-year OS: 75% vs. 83%), suggesting no benefit from chemotherapy (81). In patients with MSI-high GC, the role of perioperative or adjuvant chemotherapy remains controversial. The high mutation rate in MSI-high tumors, amends them more sensitive to immunotherapy and is an area of ongoing investigation (74,82), with some very exciting data emerging suggesting excellent pathological response with combined programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA4) blockade in the neoadjuvant setting (83). These promising response data await survival outcomes prior to be applied routinely in this disease.
Programmed cell death 1 ligand 1 (PD-L1)
More than 40% of human GC samples express PD-L1 (84). Accumulating evidence indicates that immune checkpoint inhibition (ICI) in combination with chemotherapy improve outcomes in patients with advanced or metastatic PD-L1 GC (85). A perioperative regimen of docetaxel-based chemotherapy in combination with PD-L1 blockade is investigated in two ongoing phase III trials. The MATTERHORN study examining neoadjuvant durvalumab (an anti-PD-L1 antibody) and FLOT chemotherapy followed by adjuvant durvalumab monotherapy in patients with resectable gastric/GEJ cancer (86). KEYNOTE-585 randomized patients with locally-advanced gastric or GEJ adenocarcinoma to perioperative chemotherapy with or without pembrolizumab (anti-PD-1) (87).
Epstein-Barr virus
EBV-positive GC accounts for 2–20% of total GC cases and is probably affected by environmental and geographical factors as it is slightly less prevalent in Asians than in Caucasians (88,89). EBV-positive GC occurs mostly in young males and localizes to the proximal stomach (88,89). EBV-positive tumours have a strong immunogenic pattern with PD-L1 expression, making them good candidate for PD-L1 blockade therapy (89,90). Several reports have demonstrated an intense response of EBV-positive GC to ICI (91); however, prospective data is still lacking. The ongoing French IMHOTEP phase II trial (NCT04795661) is assessing the role of pembrolizumab in the neoadjuvant setting for EBV-positive or MSI high GC (92). Table 2 summarizes selected targeted therapies and biomarker-directed trials.
Table 2
| Trial | Population/target | Investigational arm | Control arm | Results |
|---|---|---|---|---|
| INNOVATION (ongoing phase II) | HER2-positive resectable gastric or GEJ ADC | Arm I: perioperative chemotherapy + trastuzumab; Arm II: perioperative chemotherapy + trastuzumab+ pertuzumab (chemotherapy of choice: FLOT, FOLFOX, CapOx) | Perioperative chemotherapy | Pending |
| NEONIPIGA (phase II) | Locally-advanced resectable dMMR/MSI-H gastric or GEJ ADC | Neoadjuvant nivolumab and ipilimumab followed by surgery and adjuvant nivolumab | Single arm | 59% pCR |
| IMHOTEP (ongoing phase II) | Resectable dMMR/MSI-H gastric adenocarcinomas (colorectal, endometrial, other*) or EBV+ gastric cancers | Neoadjuvant single dose pembrolizumab followed by surgery and adjuvant pembrolizumab for 1 year in the absence of disease progression | Single arm | Pending |
| KEYNOTE-585 (ongoing phase III) |
Immunotherapy in the perioperative treatment for patients with resectable gastric or GEJ ADC | Pembrolizumab plus chemotherapy as neoadjuvant treatment; pembrolizumab plus chemotherapy as adjuvant treatment followed by pembrolizumab monotherapy (chemotherapy of choice: XP, FP, FLOT) | Placebo plus chemotherapy as neoadjuvant treatment; placebo plus chemotherapy as adjuvant treatment followed by placebo monotherapy (chemotherapy of choice: XP, FP, FLOT) | Pending |
| MATTERHORN (ongoing phase III) | Immunotherapy in the perioperative treatment for patients with resectable gastric or GEJ ADC | Durvalumab and FLOT chemotherapy, followed by adjuvant durvalumab monotherapy | Placebo plus chemotherapy followed by adjuvant placebo monotherapy | Pending |
other*, biliary tract or pancreas adenocarcinoma and small bowel adenocarcinoma. HER2, human epidermal growth factor receptor 2; GEJ, gastroesophageal junction; ADC, adenocarcinoma; FLOT, 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel; FOLFOX, 5-fluorouracil/leucovorin, oxaliplatin; CapOx, capecitabine, oxaliplatin; dMMR, deficient mismatch repair; MSI-H, microsatellite instability-high; ADC, adenocarcinoma; pathological complete response; EBV, Epstein-Barr virus; XP, capecitabine, cisplatin; FP, fluorouracil, cisplatin; pCR, pathological complete response.
Metastatic GC
The treatment of advanced/metastatic GC is composed of several cytotoxic agents and targeted therapies, whereas surgery is usually reserved for palliation of tumor-related symptoms such as bleeding and obstruction (93). The goals of treatment in the metastatic setting are palliative in intent and focused on improving the quality of life and prolongation of life. Treatment decisions are made based on the patient’s performance status, co-morbidities, and the regimen’s toxicity profile. In addition to improving symptoms and disease burden, systemic chemotherapy increases OS to an average of 12 months compared with 4 months with supportive care alone (94,95). When compared to single-agent chemotherapy, combination chemotherapy usually results in better response rates and longer survival times (94). Though there is no standard first-line therapy, a two-drug regimen of fluoropyrimidine and platinum is usually the selected regimen for most patients, with oxaliplatin mostly preferred over cisplatin due to lesser toxicity and similar effectiveness (96). A three-drug regimen is usually reserved for patients with good performance status who are medically fit. Docetaxel-bases triplet was shown to provide a higher response rate and longer PFS, which was counterbalanced by increased toxicity (97). ToGA (Trastuzumab for Gastric Cancer) demonstrated moderately but significantly improved outcomes for patients with advanced GC who are HER2-positive. Trastuzumab added to platinum-based chemotherapy (capecitabine plus cisplatin or fluorouracil plus cisplatin) resulted in a median OS of 13.8 months as compared to 11.1 months with chemotherapy alone (66). In the CheckMate 649 trial, nivolumab (a monoclonal antibody to PD-1) and ipilimumab (a recombinant antibody to CTLA4) were studied for patients with HER2 negative advanced GC (85). A total of 1,581 patients with gastric, GEJ or esophageal cancer, previously untreated, advanced non-resectable and HER2-negative were randomized to either nivolumab plus chemotherapy (capecitabine with oxaliplatin or fluorouracil with leucovorin with oxaliplatin), chemotherapy alone, or nivolumab plus ipilimumab. At 13.1-month follow-up, a statistically significant improvement in OS was observed when nivolumab was added to standard chemotherapy (13.8 vs. 11.6 months). The largest OS benefits were observed in patients with a Combined Positive Score (CPS) score of ≥5 and ≥1 (14.4 vs. 11.1 months and 14 vs. 11.3 months, respectively). In patients with advanced HER2-negative GC and CPS ≥ 5, the treatment of choice in first line is nivolumab combined with chemotherapy (fluoropyrimidine and oxaliplatin) (12).
In the 2nd line therapy, the preferred treatment is depended upon prior therapy and performance status, and to test regimens that haven’t yet been tried as a first line of treatment. Ramucirumab as monotherapy or combined with paclitaxel has been investigated as a second-line therapy. In the phase 3 REGARD trial, ramucirumab was superior to placebo as a second-line treatment for advanced GC and provided a modest but significant 1.4-month survival benefit (98). Patients who progressed on first-line chemotherapy were randomized to either paclitaxel and ramucirumab or placebo in the RAINBOW trial (68). The combination of ramucirumab and paclitaxel significantly improved PFS and OS (4.4 vs. 2.9 months and 9.6 vs. 7.4 months, respectively). Among patients who have good performance status, this combination therapy is the second-line treatment of choice for those who progressed on a fluoropyrimidine and platinum. A third-line regimen that is now approved is the orally fluoropyrimidine combination Trifluridine/tipiracil, that showed a survival benefit compared with placebo in the TAGS phase III trial (5.7 vs. 3.6 months) (99). In spite of a high rate of serious adverse events (43%), the addition of this drug has allowed for prolongation of life for patients who otherwise received supportive care only. For patients with MSI-H GC who progressed on previous therapies, pembrolizumab is a valid option who demonstrated 45.8% objective response rate and 11 months of PFS in the KEYNOTE-158 trial (100).
An emerging promising target for advanced GC is the fibroblast growth factor receptor (FGFR). In a recent phase II trial investigating bemarituzumab, a monoclonal antibody for FGFR2b receptor, patients were screened for the FGFR2b status and randomized to either bemarituzumab or placebo; all patients received chemotherapy (oxaliplatin, leucovorin, 5-FU) (101). Patients treated with the combination of chemotherapy plus bemarituzumab had favorable PFS (9.5 vs. 7.4 months, P=0.073) compared to chemotherapy alone. In spite the lack of significant statistical difference in PFS, bemarituzumab showed encouraging clinical effect and a phase 3 trial is underway.
Novel approaches to guide treatment
Clinical and pathological response are not always good surrogates for long-term outcomes, and various emerging other techniques are under investigation to guide pre- and postoperative therapy. Following therapy, circulating tumor DNA (ctDNA) can be measured in the blood and used to diagnose minimal residual disease (MRD). The persistence of ctDNA after surgical resection was shown to predict survival outcomes in various solid malignancies (51,102). In locoregional GC patients treated with curative intent, ctDNA identified patients at high risk for recurrence before the radiographic recurrence occurred (103). In a cohort of 1,630 gastroesophageal adenocarcinoma patients, ctDNA characterized genomic alterations that correlated with clinicopathologic characteristics and outcomes (104).
Conclusions
Despite recent advancements in systemic therapy and improvements in outcomes, gastric adenocarcinoma remains a leading cause of death worldwide. Early detection offers the best chances of survival, with continued research needed at developing novel less invasive tools for early detection. The combination of surgical resection and perioperative systemic therapy improves long-term outcomes in patients with locally advanced gastric adenocarcinoma. Ongoing studies are aimed at optimizing perioperative and systemic therapies, as well as incorporating checkpoint inhibitors and biomarker-directed therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francisco Schlottmann and Marco G. Patti) for the series “Current Management of Upper Gastrointestinal Malignancies” published in Journal of Gastrointestinal Oncology. The article has undergone external peer review.
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-818/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-818/coif). The series “Current Management of Upper Gastrointestinal Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol 2022;28:1187-203. [Crossref] [PubMed]
- Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer 1990;62:440-3. [Crossref] [PubMed]
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53. [Crossref] [PubMed]
- Dikken JL, Lemmens VE, Wouters MW, et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer 2012;48:1624-32. [Crossref] [PubMed]
- Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019;4:e137-47. [Crossref] [PubMed]
- Arnold M, Park JY, Camargo MC, et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020;69:823-9. [Crossref] [PubMed]
- Heer EV, Harper AS, Sung H, et al. Emerging cancer incidence trends in Canada: The growing burden of young adult cancers. Cancer 2020;126:4553-62. [Crossref] [PubMed]
- In H, Solsky I, Palis B, et al. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol 2017;24:3683-91.
- SEER. Stomach. Stage Distribution of SEER Incidence Cases, 2011-2020. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=18&data_type=1&graph_type=4&compareBy=sex&chk_sex_1=1&race=1&age_range=1&advopt_precision=1&advopt_display=2
- Chan WL, Lam KO, Lee VHF, et al. Gastric Cancer - From Aetiology to Management: Differences Between the East and the West. Clin Oncol (R Coll Radiol) 2019;31:570-7. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Gastric Cancer (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Yang K, Lu L, Liu H, et al. A comprehensive update on early gastric cancer: defining terms, etiology, and alarming risk factors. Expert Rev Gastroenterol Hepatol 2021;15:255-73. [Crossref] [PubMed]
- Youn JC, Youn YH, Kim TI, et al. Factors affecting long-term clinical outcomes of endoscopic mucosal resection of early gastric cancer. Hepatogastroenterology 2006;53:643-7. [PubMed]
- Uedo N, Iishi H, Tatsuta M, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer 2006;9:88-92. [Crossref] [PubMed]
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [Crossref] [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25. [Crossref] [PubMed]
- Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc 2007;66:693-700. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Hoteya S, Iizuka T, Kikuchi D, et al. Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol 2009;24:1102-6. [Crossref] [PubMed]
- Uedo N, Takeuchi Y, Ishihara R. Endoscopic management of early gastric cancer: endoscopic mucosal resection or endoscopic submucosal dissection: data from a Japanese high-volume center and literature review. Ann Gastroenterol 2012;25:281-90. [PubMed]
- Park YM, Cho E, Kang HY, et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc 2011;25:2666-77. [Crossref] [PubMed]
- Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009;41:746-50. [Crossref] [PubMed]
- Watanabe T, Kume K, Taip M, et al. Gastric mucosal cancer smaller than 7mm can be treated with conventional endoscopic mucosal resection as effectively as with endoscopic submucosal dissection. Hepatogastroenterology 2010;57:668-73. [PubMed]
- Najmeh S, Cools-Lartigue J, Mueller C, et al. Comparing Laparoscopic to Endoscopic Resections for Early Gastric Cancer in a High Volume North American Center. J Gastrointest Surg 2016;20:1547-53. [Crossref] [PubMed]
- Jeon HK, Kim GH, Lee BE, et al. Long-term outcome of endoscopic submucosal dissection is comparable to that of surgery for early gastric cancer: a propensity-matched analysis. Gastric Cancer 2018;21:133-43. [Crossref] [PubMed]
- Abdelfatah MM, Barakat M, Ahmad D, et al. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2019;31:418-24. [Crossref] [PubMed]
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016;19:198-205. [Crossref] [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc 2021;33:4-20.
- Ngamruengphong S, Ferri L, Aihara H, et al. Efficacy of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasia in a Large Cohort in North America. Clin Gastroenterol Hepatol 2021;19:1611-1619.e1. [Crossref] [PubMed]
- Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Al-Batran SE, Lorenzen S. Management of Locally Advanced Gastroesophageal Cancer: Still a Multidisciplinary Global Challenge? Hematol Oncol Clin North Am 2017;31:441-52. [Crossref] [PubMed]
- Cai Z, Yin Y, Shen C, et al. Comparative effectiveness of preoperative, postoperative and perioperative treatments for resectable gastric cancer: A network meta-analysis of the literature from the past 20 years. Surg Oncol 2018;27:563-74. [Crossref] [PubMed]
- Coccolini F, Nardi M, Montori G, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg 2018;51:120-7. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Maspero M, Sposito C, Benedetti A, et al. Impact of Surgical Margins on Overall Survival after Gastrectomy for Gastric Cancer: A Validation of Japanese Gastric Cancer Association Guidelines on a Western Series. Ann Surg Oncol 2022;29:3096-108. [Crossref] [PubMed]
- Lee HJ, Hyung WJ, Yang HK, et al. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg 2019;270:983-91. [Crossref] [PubMed]
- Hyung WJ, Yang HK, Park YK, et al. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol 2020;38:3304-13. [Crossref] [PubMed]
- van der Veen A, Brenkman HJF, Seesing MFJ, et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J Clin Oncol 2021;39:978-89. [Crossref] [PubMed]
- Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 1988;75:110-2. [Crossref] [PubMed]
- Robertson CS, Chung SC, Woods SD, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg 1994;220:176-82. [Crossref] [PubMed]
- Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995;345:745-8. [Crossref] [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995-9. [Crossref] [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg 2004;91:283-7. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [PubMed]
- Karavokyros I, Michalinos A. Favoring D(2)-Lymphadenectomy in Gastric Cancer. Front Surg 2018;5:42. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Nevo Y, Goldes Y, Barda L, et al. Risk Factors for Complications of Total/Subtotal Gastrectomy for Gastric Cancer: Prospectively Collected, Based on the Clavien-Dindo Classification System. Isr Med Assoc J 2018;20:277-80. [PubMed]
- Jiang L, Yang KH, Guan QL, et al. Survival and recurrence free benefits with different lymphadenectomy for resectable gastric cancer: a meta-analysis. J Surg Oncol 2013;107:807-14. [Crossref] [PubMed]
- Lumish MA, Ku GY. Approach to Resectable Gastric Cancer: Evolving Paradigm of Neoadjuvant and Adjuvant Treatment. Curr Treat Options Oncol 2022;23:1044-58. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. [Crossref] [PubMed]
- Yoshida K, Kodera Y, Kochi M, et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol 2019;37:1296-304. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Park SH, Lim DH, Sohn TS, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial. Ann Oncol 2021;32:368-74. [Crossref] [PubMed]
- Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:616-28. [Crossref] [PubMed]
- Stiekema J, Trip AK, Jansen EP, et al. Does adjuvant chemoradiotherapy improve the prognosis of gastric cancer after an r1 resection? Results from a dutch cohort study. Ann Surg Oncol 2015;22:581-8. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol 2017;18:357-70. [Crossref] [PubMed]
- Al-Batran SE, Hofheinz RD, Schmalenberg H, et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7): Results of the phase II-portion—A multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. J Clin Oncol 2020;38:abstr 4501.
- Hofheinz RD, Haag GM, Ettrich TJ, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: Final results of the PETRARCA multicenter randomized phase II trial of the AIO. J Clin Oncol 2020;38:abstr 4502.
- Hofheinz RD, Hegewisch-Becker S, Kunzmann V, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer 2021;149:1322-31. [Crossref] [PubMed]
- Wagner AD, Grabsch HI, Mauer M, et al. EORTC-1203-GITCG - the "INNOVATION"-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer 2019;19:494. [Crossref] [PubMed]
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Ratti M, Lampis A, Hahne JC, et al. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci 2018;75:4151-62. [Crossref] [PubMed]
- Kim JY, Shin NR, Kim A, et al. Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol 2013;47:28-35. [Crossref] [PubMed]
- Puliga E, Corso S, Pietrantonio F, et al. Microsatellite instability in Gastric Cancer: Between lights and shadows. Cancer Treat Rev 2021;95:102175. [Crossref] [PubMed]
- Polom K, Marano L, Marrelli D, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018;105:159-67. [Crossref] [PubMed]
- Zubarayev M, Min EK, Son T. Clinical and molecular prognostic markers of survival after surgery for gastric cancer: tumor-node-metastasis staging system and beyond. Transl Gastroenterol Hepatol 2019;4:59. [Crossref] [PubMed]
- Pietrantonio F, Miceli R, Raimondi A, et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J Clin Oncol 2019;37:3392-400. [Crossref] [PubMed]
- Chao J, Fuchs CS, Shitara K, et al. Pembrolizumab (pembro) in microsatellite instability-high (MSI-H) advanced gastric/gastroesophageal junction (G/GEJ) cancer by line of therapy. J Clin Oncol 2020;38:abstr 430.
- André T, Tougeron D, Piessen G, et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J Clin Oncol 2023;41:255-65. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Janjigian YY, Van Cutsem E, Muro K, et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol 2022;18:2465-73. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Fuchs CS, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol 2019;15:943-52. [Crossref] [PubMed]
- Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer Int J Oncol 2015;46:1421-34. (review). [Crossref] [PubMed]
- Saito M, Kono K. Landscape of EBV-positive gastric cancer. Gastric Cancer 2021;24:983-9. [Crossref] [PubMed]
- Derks S, Liao X, Chiaravalli AM, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget 2016;7:32925-32. [Crossref] [PubMed]
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449-58. [Crossref] [PubMed]
- Suwaidan AA, Gordon A, Cartwright E, et al. Optimising Multimodality Treatment of Resectable Oesophago-Gastric Adenocarcinoma. Cancers (Basel) 2022;14:586. [Crossref] [PubMed]
- Nevo Y, Morency D, Kammili A, et al. The Role of Palliative Surgery in Stage IV Gastric Cancer: A Retrospective Study. J Palliat Care 2022;37:152-8. [Crossref] [PubMed]
- Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064. [PubMed]
- Cann C, Ciombor KK. Systemic therapy for gastric cancer: Perioperative strategies and beyond. J Surg Oncol 2022;125:1151-60. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Al-Batran SE, Pauligk C, Homann N, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer 2013;49:835-42. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:1437-48. [Crossref] [PubMed]
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2020;38:1-10. [Crossref] [PubMed]
- Wainberg ZA, Enzinger PC, Kang YK, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 2022;23:1430-40. [Crossref] [PubMed]
- Hsieh CC, Hsu HS, Chang SC, et al. Circulating Cell-Free DNA Levels Could Predict Oncological Outcomes of Patients Undergoing Esophagectomy for Esophageal Squamous Cell Carcinoma. Int J Mol Sci 2016;17:2131. [Crossref] [PubMed]
- Yang J, Gong Y, Lam VK, et al. Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis 2020;11:346. [Crossref] [PubMed]
- Maron SB, Chase LM, Lomnicki S, et al. Circulating Tumor DNA Sequencing Analysis of Gastroesophageal Adenocarcinoma. Clin Cancer Res 2019;25:7098-112. [Crossref] [PubMed]





