
The safety and effects of high- and low-volume polyethylene glycol bowel preparation methods before colonoscopy on bowel cleanliness: a systematic review and meta-analysis
Highlight box
Key findings
• In clinical practice, considering patient compliance and safety, a low-dose PEG mixed solution regimen may be a more optimal option.
What is known and what is new?
• Some studies support the low-volume PEG method and believe that its effectiveness is no less than that of traditional methods, while others believe that its effectiveness is poor. This situation makes clinical doctors confused when actually choosing intestinal cleaning methods.
• We use meta-analysis to analyze the impact of current intervention methods on intestinal cleanliness and patient safety, in order to find more suitable intestinal cleaning methods.
What is the implication, and what should change now?
• Although the high-volume PEG and low-volume PEG mixed solution have similar effects on intestinal cleanliness, considering that the low dose combination scheme is more favored by patients and has a lower incidence of adverse reactions, it is recommended to prioritize the low-volume PEG mixed solution in clinical practice.
Introduction
Colonoscopy is one of the most important diagnostic and therapeutic methods for colon diseases (1-3). Colonoscopy is also widely recommended for colorectal cancer screening and surveillance and has been reported to reduce colorectal cancer mortality by approximately 60% (4). Adequate bowel preparation is essential for colonoscopy. The success of a colonoscopy depends on high-quality bowel preparation that enables the clear visualization of the intestinal mucosa, facilitates a smooth diagnosis and treatment, and minimizes the risk of contamination (5,6). High-quality colonoscopy is increasingly associated with a good prognosis in patients with colorectal cancer (7).
The fractionated drinking plan of high capacity (4 L) polyethylene glycol (PEG) protocol has become the gold standard for bowel preparation (8). However, as the patients are required to drink a large amount of PEG solution, their compliance and acceptance of this method are poor and their willingness to reuse the method is low (9-11). Thus, the solution for reducing high-volume PEG still needs to be improved.
A low-volume PEG bowel preparation was developed that halved the original volume of 4 to 2 L, and added some bowel preparation aids, such as citrate and simethicone, to improve tolerance, acceptability, and compliance (12-14). However, there is currently no consensus as to whether the addition of adjuvant drugs to low-volume PEG achieves good intestinal cleanliness. The fundamental reason for this controversy is that different clinical studies often draw different or even contradictory conclusions. Some studies support the low-volume PEG method and believe that its effectiveness is no less than that of traditional methods, while others believe that its effectiveness is poor. This situation makes clinical doctors confused when actually choosing intestinal cleaning methods. Thus, we conducted a meta-analysis of relevant articles to evaluate the effects of the current intervention methods on intestinal cleanliness and patient safety. We present this article in accordance with the PRISMA reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-581/rc).
Methods
Document retrieval
The PubMed, EMBASE, and Cochrane Library databases were searched to retrieve high-quality articles on low-volume mixed and high-volume bowel preparation methods before colonoscopy published from the inception of the databases to October 15, 2022. The search language was limited to English, and the search keywords included low-volume, high-volume, colonoscopy, bowel preparation, PEG solutions, adverse effects, drug therapy, patient compliance, and patient satisfaction. For example, the following searches were conducted in the PubMed databases: (I) bowel preparation AND colonoscopy; (II) PEG solutions AND low-volume OR high-volume OR drug therapy; (III) adverse effects OR patient compliance OR patient satisfaction; (IV) I AND II AND III.
Literature inclusion and exclusion criteria
To be eligible for inclusion in this meta-analysis, the articles had to meet the following inclusion criteria: (I) research design: randomized controlled trial (RCT). The research objects were adult patients aged ≥18 years requiring bowel preparation. (II) Interventions: in relation to the bowel preparation method, in the low-volume PEG mixed group, an adjunct drug was added to the 2 L of PEG solution, while in the high-volume group, the standard 4 L of PEG solution was administered in split doses of 2 L + 2 L or 3 L + 1 L. Patients with any underlying conditions were included. (III) Outcomes in the literature, the seven main outcome indicators were: (i) the total score of the Boston Bowel Preparation Scale (BBPS) or Ottawa Bowel Preparation Scale (OBPS); (ii) the qualified rate of intestinal cleanliness; (iii) the repeat willingness rate; (iv) patient compliance; (v) nausea; (vi) vomiting; and (vii) abdominal pain.
Articles were excluded from the meta-analysis if they met any of the following exclusion criteria: (I) concerned a review, news, animal experiment, scoring, dissertations or meta-analysis; (II) used interventions that did not meet the set standards; (III) contained incomplete data or data that could not be extracted; and/or (IV) were duplicate articles or repeat publications by the same author/s.
Literature screening and data extraction
According to the inclusion and exclusion criteria, the two authors independently searched the above-mentioned databases and extracted the data according to the search formula. The data mainly included general information (e.g., the author, publication year, country, and intervention measures) and the seven main outcome indicators.
Literature quality evaluation
The Cochrane Handbook for Systematic Reviews of Interventions (https://china.cochrane.org/) was used to evaluate the quality of the final included studies, and the two researchers reviewed the full text of the articles to evaluate the quality of the articles. Two researchers evaluated the randomization methods, allocation concealment, double blinding, data integrity, selection reports, and other possible biases for each study based on the recommendations in the manual, and determined them to be “low risk”, “unclear risk”, or “high risk”. If all items are low risk, it means low bias of risk, if some are uncertain risk, it means unclear risk of bias, and if all items are high risk, it means high bias of risk.
Statistical analysis
R 4.2.1 software (Lucent Technologies, USA) was used for the meta-analysis. The binary variables are expressed as the risk ratio (RR) with a 95% confidence interval (CI). The continuous variables are expressed as the weighted mean difference (WMD) and 95% CI. I2 was used to test for heterogeneity. If I2≥50%, there was obvious heterogeneity among the included studies, and a random-effects model was used. If I2<50%, the heterogeneity among included studies was relatively small and acceptable, and a fixed-effects model was used. A funnel plot test was used for publication bias analysis, and a bilateral P<0.05 was considered statistically significant.
Results
Literature search results
Using the above-mentioned search method, a total of 2,635 eligible articles were initially retrieved. After excluding duplicates and articles with incomplete information, 2,103 articles remained. After reading the titles and abstracts and removing articles that did not match the theme, or were reviews, news, etc., 120 articles remained. The full text of these articles was then downloaded. After the full-text review, 15 articles were included in the meta-analysis. The literature screening process is shown in Figure 1, and the basic characteristics of the included articles are shown in Table 1.
Table 1
| Study (author year) | Country | Participants | Age (years), mean ± SD | High-volume | Low-volume | Conclusions |
|---|---|---|---|---|---|---|
| Barkun 2022 (15) | Canada | 2,314 | 56.3±13.0 | 2 L + 2 L of PEG | 1 L + 1 L of PEG + bisacodyl (15 mg) | 1, 2, 3, 4, 5, 6, 7 |
| Cesaro 2013 (12) | Italy | 101 | 57.7±9.4 | 3 L + 1 L of PEG | 2 L of PEG + bisacodyl (2–4 tablets) | 1, 2, 3, 4, 5, 6, 7 |
| Corporaal 2010 (13) | Netherlands | 307 | 54±14 | 2 L + 2 L of PEG | 2 L of PEG + 200 g of Asc | 2, 5, 6, 7 |
| Gimeno-García 2017 (16) | Spain | 256 | 64.3±13.63 | 2 L + 2 L of PEG | 2 L of PEG + 4 sachets of Asc | 1, 3 |
| Jansen 2011 (9) | Netherlands | 370 | 58.6±14.2 | 2 L + 2 L of PEG | 2 L of PEG + Asc | 2, 4, 7 |
| Jung 2016 (14) | Korea | 130 | 71.15±4.5 | 2 L + 2 L of PEG (250 mL every 15 min) | 1 L of PEGA + 1 L of PEGA (250 mL every 15 min) | 1, 2, 3, 5, 6, 7 |
| Kim 2016 (10) | Korea | 319 | 46.5±9.75 | 2 L + 2 L of PEG (250 mL every 15 min) | 2 L of PEG + 250 mL of Asc | 3, 4 |
| Marmo 2010 (17) | Italy | 435 | 58.3±14.8 | 2 L + 2 L of PEG | 2 L of PEG + Asc | 2, 5, 6 |
| Mathus-Vliegen 2013 (18) | UK | 188 | 59.6±13.1 | 2 L + 2 L of PEG | 2 L of PEG + Asc | 1, 3, 5, 6, 7 |
| Moon 2014 (11) | Korea | 327 | 53.1±11.7 | 2 L + 2 L of PEG | 2 L of PEG + Asc | 4, 5, 6, 7 |
| Mussetto 2015 (19) | Italy | 120 | 66.9±9.8 | 2 L + 2 L of PEG | 2 L of PEG + 15 mg of bisacodyl | 1, 2 |
| Parente 2015 (20) | Italy | 382 | 59.5±13.5 | 2 L + 2 L of PEG | 2 L of PEG + 15 mg of bisacodyl | 1,2 |
| Rodríguez 2015 (21) | Spain | 194 | 62.25±5.4 | 2 L + 2 L of PEG | 2 L of PEG + Asc | 1, 3, 4, 5, 6, 7 |
| Sirinawasatien 2022 (22) | Thailand | 140 | 58.7±10.8 | 2 L + 2 L of PEG (250 mL) | 2 L of PEG + 24 µg of lubiprostone | 1, 2, 3, 4, 5, 6, 7 |
| Valiante 2013 (23) | Italy | 264 | 62.4±7.4 | 3 L + 1 L | 2 L of PEG + 15 mg of bisacodyl | 2, 3, 4, 5, 7 |
Conclusions: 1. BBPS or OBPS total score; 2. bowel cleanliness pass rate; 3. patient willingness to repeat; 4. patient compliance; 5. nausea; 6. vomiting; 7. abdominal pain. SD, standard deviation; PEG, polyethylene glycol; Asc, ascorbic acid; BBPS, Boston Bowel Preparation Scale; OBPS, Ottawa bowel preparation scale.
The results of the quality evaluation of the included literature
The 15 included articles all concerned RCTs. The quality of the literature was evaluated using the method recommended by the Cochrane manual (https://training.cochrane.org/handbook/current). If ≥1 of the seven items was rated as unclear and/or high risk, the literature had unclear risk of bias. If all seven items are low risk, then the literature has a low risk of bias. The results are shown in Figure 2.
Bowel cleanliness BBPS/OBPS scores
A meta-analysis of the BBPS/OBPS scores of bowel cleanliness was performed using data from 9 of the included RCTs. Based on the heterogeneity results (I2=96%), there was high heterogeneity among the included studies. The source of heterogeneity was analyzed and found to be the study of Mathus-Vliegen et al. (18). The meta-analysis showed that the high-volume PEG group had a higher intestinal cleanliness score than the low-volume PEG mixed solution group [mean difference (MD) =0.09, 95% CI: –0.29 to 0.46, P=0.65], and the difference was not statistically significant (Figure 3).

Qualified rate of intestinal cleanliness
In total, 10 studies examined the pass bowel cleansing rate in the high-volume PEG and low-volume PEG mixed solution groups. Based on the heterogeneity test results (I2=75%), there was moderate heterogeneity among the included studies. Thus, the random-effects model was used to analyze the source of heterogeneity, which appeared to be the study of Cesaro et al. (12). The meta-analysis showed that the high-volume PEG group had a higher qualified bowel cleansing rate than the low-volume PEG mixed solution group (RR =1.02, 95% CI: 0.94–1.11, P=0.59), and the difference was not statistically significant (Figure 4).
Repeat willingness rate among patients
In total, 9 studies compared the patients’ willingness to repeat the bowel cleansing methods in the high-volume PEG and low-volume PEG groups. Based on the heterogeneity test results (I2=89%), the random-effects model was used. The source of heterogeneity appeared to be the study of Cesaro et al. (12). The meta-analysis showed that patients in the low-volume PEG mixed solution group had a higher repeat willingness of bowel cleansing rate than those in the high-volume PEG group (RR =0.71, 95% CI: 0.60–0.84, P<0.01) (Figure 5).

Patient compliance
In total, 8 studies compared the compliance of patients with the bowel cleansing methods in the high- and low-volume PEG groups. The heterogeneity test results were as follows: I2=90%. Again, the source of heterogeneity appeared to be the study of Cesaro et al. The meta-analysis results showed that the compliance with the bowel cleansing method of patients in the low-volume PEG mixed solution group was significantly higher compared to the high-volume PEG group (RR =0.91, 95% CI: 0.80–1.02, P=0.12), and the difference was not statistically significant (Figure 6).
Incidence of nausea
In relation to the adverse reactions, 10 studies compared the incidence of nausea in the patients who used the bowel cleansing methods in the high-volume PEG group and the low-volume PEG mixed solution group. A fixed-effects model was used. The meta-analysis showed that the incidence of nausea in the high-volume PEG group was significantly higher compared to the low-volume PEG mixed solution group (RR =1.38, 95% CI: 1.22–1.56, P<0.01) (Figure 7).
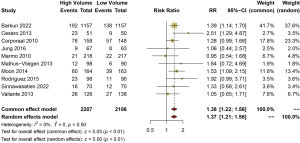
Incidence of vomiting
In total, 9 studies compared the incidence of vomiting in the high- and low-volume PEG groups. The heterogeneity test results were as follows: I2=9%. The meta-analysis showed that the incidence of vomiting in the high-volume PEG group was significantly higher compared to the low-volume PEG mixed solution group (RR =1.79, 95% CI: 1.41–2.27, P<0.01) (Figure 8).
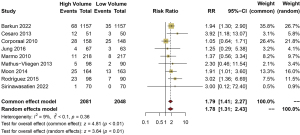
Incidence of abdominal pain
In total, 10 studies compared the incidence of abdominal pain in the high- and low-volume PEG groups. The heterogeneity test results were as follows: I2=22%. The meta-analysis showed that the incidence of nausea in the high-volume PEG group was significantly higher compared to the low-volume PEG mixed solution group (RR =1.05, 95% CI: 1.01–1.08, P<0.01) (Figure 9).

Publication deviation evaluation
A publication bias analysis was performed on the outcome indicators in the included articles (i.e., the pass bowel cleansing rate and the incidence of nausea), and a funnel plot was drawn using R 4.2.1 software (Figure 10). The funnel graph of the qualified rate of intestinal cleaning showed that two studies were outside the funnel, but still showed symmetry. The funnel graph of the incidence of nausea showed that all studies were inside the funnel and showed symmetry, indicating that the included literature publication bias was relatively small or that there was no publication bias.

Discussion
In recent years, the split dosing of the high-volume (4 L) PEG solution has been recognized as the standard protocol for bowel preparation before colonoscopy. However, patients often report that drinking the high-volume PEG solution leads to adverse reactions, such as nausea, vomiting, abdominal pain, and sleep disturbance (21,24), which in turn leads to a decrease in compliance among many patients. The discomfort symptoms of patients lead to interruptions in drinking, and the colonoscopies of such patients do not progress smoothly (19,25). Such bad experiences also cause patients to reject the method and reduce their willingness to repeatedly use high volume PEG methods (12,15,16). To improve the compliance of patients with this preparation, some studies have recommended that other drugs, such as linaclotide or prucalopride, be added to a 2 L, low-volume PEG enteral formulation to reduce the solution volume (15,26) and thus achieve a good bowel cleansing rate and increase patient compliance.
In this study, the patients who received the PEG-based regimens were divided into the following two groups based on their PEG intake and the addition of adjunctive laxatives: (I) the low-volume (2 L) PEG mixed solution group (which also received additional adjuvant drugs); and (II) the high-volume (4 L) PEG group. Although the two methods have similar results in cleanliness, and there is no difference in intestinal cleanliness score and intestinal cleanliness rate (P>0.05), which is consistent with the results of some studies (9,15), patients have a higher acceptance of low-volume PEG mixed regimen. The use of a low-volume PEG mixed adjuvant approach can also achieve a good bowel cleansing pass rate. Further, the repeat willingness rate with the bowel cleansing for patients in the low-volume PEG mixed solution group were much higher than those in the high-volume PEG group (P<0.01); that is, the acceptance of patients in the low-volume group was better than that of patients in the high-volume group.
In addition, we also observed that the incidence of adverse reactions in the high-volume PEG group was significantly higher than that in the low-volume PEG mixed solution group. Especially, the symptoms of nausea, vomiting, and abdominal pain increased significantly (P<0.01). These adverse reactions not only affect the patient’s experience, but may also further reduce their compliance with intestinal preparation. Therefore, in addition to considering intestinal cleanliness, reducing the occurrence of adverse reactions should also be considered a key factor in selecting intestinal preparation methods. Thus, the use of the low-volume PEG mixed solution with adjuvant drugs as the bowel preparation method for clinical colonoscopy appears to improve patients’ medication experience and compliance.
This study had some limitations. Notably, there was heterogeneity in some of the results. The source of the heterogeneity in the analysis may be related to the low number of included samples (i.e., only 15 studies were included in the meta-analysis) or may be because the adjuvant drugs used in the low-volume PEG groups differed between the studies. Additionally, the dosages of the drugs also differed, but due to the limited number of studies and the unknown dosages of the adjuvant drugs in some studies, further subgroup analyses could not be performed. Thus, the effects of low-volume PEG plus adjuvant drugs on the safety of intestinal cleanliness needs to be examined in future studies. More articles are needed for a more detailed analysis.
Conclusions
Although high-volume PEG and low-volume PEG mixed solution regimens have similar effects on intestinal cleanliness, considering that low-volume PEG mixed solution regimens are more favored by patients and have a lower incidence of adverse reactions, it is recommended to prioritize low-volume PEG mixed solution regimens in clinical practice, especially for patients who may be at risk of adverse reactions. Considering the limitations of this article, more high-quality research should be included for verification in the future.
Acknowledgments
We thank Dr. Christian S. Jackson (Loma Linda VA Healthcare System, Loma Linda, CA, USA), Dr. Benedicte Schelde-Olesen (Odense University Hospital, Denmark) for the critical comments and valuable advice on this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-581/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-581/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-581/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halpern Z, Gross SA, Gralnek IM, et al. Comparison of adenoma detection and miss rates between a novel balloon colonoscope and standard colonoscopy: a randomized tandem study. Endoscopy 2015;47:238-44. [Crossref] [PubMed]
- Enestvedt BK, Tofani C, Laine LA, et al. 4-Liter split-dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta-analysis. Clin Gastroenterol Hepatol 2012;10:1225-31. [Crossref] [PubMed]
- Agrawal R, Majeed M, Attar BM, et al. Predictors of poor bowel preparations and colonoscopy cancellations in inpatient colonoscopies, a single center retrospective study. Transl Gastroenterol Hepatol 2022;7:4. [Crossref] [PubMed]
- Pan J, Xin L, Ma YF, et al. Colonoscopy Reduces Colorectal Cancer Incidence and Mortality in Patients With Non-Malignant Findings: A Meta-Analysis. Am J Gastroenterol 2016;111:355-65. [Crossref] [PubMed]
- Gao X, Bian Q, Ding W, et al. Effect of Walking Exercise and Intestinal Cleansing Interval on Bowel Preparation Quality, a Single-Blind, Randomized Controlled Trial. Dig Dis Sci 2023;68:193-201. [Crossref] [PubMed]
- Hernandez PV, Horsley-Silva JL, Snyder DL, et al. Effect of bowel preparation volume in inpatient colonoscopy. Results of a prospective, randomized, comparative pilot study. BMC Gastroenterol 2020;20:227. [Crossref] [PubMed]
- Liu X, Yuan M, Li Z, et al. The Efficacy of Simethicone With Polyethylene Glycol for Bowel Preparation: A Systematic Review and Meta-Analysis. J Clin Gastroenterol 2021;55:e46-55. [Crossref] [PubMed]
- Rostom A, Dube C, Bishay K, et al. A randomized clinical prospective trial comparing split-dose picosulfate/ magnesium citrate and polyethylene glycol for colonoscopy preparation. PLoS One 2019;14:e0211136. [Crossref] [PubMed]
- Jansen SV, Goedhard JG, Winkens B, et al. Preparation before colonoscopy: a randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol 2011;23:897-902. [Crossref] [PubMed]
- Kim MS, Park J, Park JH, et al. Does Polyethylene Glycol (PEG) Plus Ascorbic Acid Induce More Mucosal Injuries than Split-Dose 4-L PEG during Bowel Preparation? Gut Liver 2016;10:237-43. [Crossref] [PubMed]
- Moon CM, Park DI, Choe YG, et al. Randomized trial of 2-L polyethylene glycol + ascorbic acid versus 4-L polyethylene glycol as bowel cleansing for colonoscopy in an optimal setting. J Gastroenterol Hepatol 2014;29:1223-8. [Crossref] [PubMed]
- Cesaro P, Hassan C, Spada C, et al. A new low-volume isosmotic polyethylene glycol solution plus bisacodyl versus split-dose 4 L polyethylene glycol for bowel cleansing prior to colonoscopy: a randomised controlled trial. Dig Liver Dis 2013;45:23-7. [Crossref] [PubMed]
- Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol 2010;45:1380-6. [Crossref] [PubMed]
- Jung YS, Lee CK, Eun CS, et al. Low-Volume Polyethylene Glycol with Ascorbic Acid for Colonoscopy Preparation in Elderly Patients: A Randomized Multicenter Study. Digestion 2016;94:82-91. [Crossref] [PubMed]
- Barkun AN, Martel M, Epstein IL, et al. The Bowel CLEANsing National Initiative: High-Volume Split-Dose Vs Low-Volume Split-Dose Polyethylene Glycol Preparations: A Randomized Controlled Trial. Clin Gastroenterol Hepatol 2022;20:e1469-77. [Crossref] [PubMed]
- Gimeno-García AZ, Hernandez G, Aldea A, et al. Comparison of Two Intensive Bowel Cleansing Regimens in Patients With Previous Poor Bowel Preparation: A Randomized Controlled Study. Am J Gastroenterol 2017;112:951-8. [Crossref] [PubMed]
- Marmo R, Rotondano G, Riccio G, et al. Effective bowel cleansing before colonoscopy: a randomized study of split-dosage versus non-split dosage regimens of high-volume versus low-volume polyethylene glycol solutions. Gastrointest Endosc 2010;72:313-20. [Crossref] [PubMed]
- Mathus-Vliegen EM, van der Vliet K. Safety, patient's tolerance, and efficacy of a 2-liter vitamin C-enriched macrogol bowel preparation: a randomized, endoscopist-blinded prospective comparison with a 4-liter macrogol solution. Dis Colon Rectum 2013;56:1002-12. [Crossref] [PubMed]
- Mussetto A, Frazzoni L, Paggi S, et al. Split dosing with a low-volume preparation is not inferior to split dosing with a high-volume preparation for bowel cleansing in patients with a history of colorectal resection: a randomized trial. Endoscopy 2015;47:917-24. [Crossref] [PubMed]
- Parente F, Vailati C, Bargiggia S, et al. 2-Litre polyethylene glycol-citrate-simethicone plus bisacodyl versus 4-litre polyethylene glycol as preparation for colonoscopy in chronic constipation. Dig Liver Dis 2015;47:857-63. [Crossref] [PubMed]
- Rodríguez de Miguel C, Serradesanferm A, López-Cerón M, et al. Ascorbic acid PEG-2L is superior for early morning colonoscopies in colorectal cancer screening programs: a prospective non-randomized controlled trial. Gastroenterol Hepatol 2015;38:62-70. [Crossref] [PubMed]
- Sirinawasatien A, Sakulthongthawin P, Chanpiwat K, et al. Bowel preparation using 2-L split-dose polyethylene glycol regimen plus lubiprostone versus 4-L split-dose polyethylene glycol regimen: a randomized controlled trial. BMC Gastroenterol 2022;22:424. [Crossref] [PubMed]
- Valiante F, Bellumat A, De Bona M, et al. Bisacodyl plus split 2-L polyethylene glycol-citrate-simethicone improves quality of bowel preparation before screening colonoscopy. World J Gastroenterol 2013;19:5493-9. [Crossref] [PubMed]
- Pan H, Zheng XL, Fang CY, et al. Same-day single-dose vs large-volume split-dose regimens of polyethylene glycol for bowel preparation: A systematic review and meta-analysis. World J Clin Cases 2022;10:7844-58. [Crossref] [PubMed]
- Yu H, Xu L, Yin S, et al. A Chinese survey of current practice patterns of preoperative bowel preparation in colorectal surgery. Dig Med Res 2022;5:22. [Crossref]
- Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697-706. [Crossref] [PubMed]






