
The effect of cognitive behavioral therapy on chemotherapy-induced side effects and immune function in colorectal cancer patients undergoing chemotherapy: study protocol for a randomized controlled trial
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal malignancies (1). It was the third most diagnosed cancer and the second most common cause of cancer-related death in the United States in 2020 (2). At present, the incidence of colorectal cancer ranks second among all malignant tumors in China (3). The number of CRC patients worldwide has more than doubled over the last 30 years (4). The number of new cases in 2022 is estimated to exceed 590,000 (3). Although the survival time of CRC patients has significantly increased (5), several studies suggest that the mental status of CRC patients is associated with more severe chemotherapy-induced side effects and poorer prognoses (6-8).
Increasing evidence suggested that CRC patients have a high risk of psychological disorders (9). Anxiety and depression are the most common psychological disorders in cancer patients (10). Among patients with CRC, approximately 20.4% and 31.8% of patients were diagnosed with depression and anxiety, respectively (11). The incidence of depression and anxiety has been shown to increase during the chemotherapy (12,13). Moreover, the risk of mental disorders remains higher 10 years after cancer treatment (14).
Depression and anxiety disorders among CRC patients could lead to more interruptions in tumor treatment (15,16), thus decreasing the quality of life (17) and leading to a higher mortality rate (18,19). In addition, CRC patients with depressive and/or anxiety disorders were reported to suffer from more side effects of chemotherapy (20). Bonhof et al. [2019] found that CRC patients with more severe adverse effects, such as chemotherapy-induced sensory peripheral neuropathy, reported more fatigue, anxiety, and depressive symptoms (21). Psychological intervention is one of the most important treatments for depression and anxiety in patients with CRC. Cognitive behavioral therapy (CBT) has been demonstrated to be effective in reducing depressive and anxiety symptoms in cancer patients (22,23), and alleviate fatigue in cancer patients (24). In theory, CBT can reduce the side effects of chemotherapy by reducing the level of depression and anxiety.
Moreover, previous studies have suggested that depression and anxiety are associated with immune function (25,26). Depression and anxiety have been associated with the immune response [e.g., lower natural killer cell counts (27)] and proinflammatory factors [e.g., interleukin-1β, interleukin-6, interleukin-8, and tumor necrosis factor-α (28)] in CRC patients. Psychological interventions (including CBT) can improve immune function in cancer patients (29).
However, whether CBT is associated with fewer chemotherapy-induced side effects and the improvement of immune function in CRC patients remains unclear. Therefore, we plan to conduct a randomized controlled trial (RCT) among CRC patients undergoing chemotherapy to determine whether CBT can reduce the side effects of chemotherapy and improve the immune function of CRC patients. We also try to explore the underlying biological mechanism of CBT. We hypothesize that CBT will reduce side effects related to chemotherapy and improve the immune function of CRC patients. We present this article in accordance with the SPIRIT reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-625/rc).
Methods
Study design and setting
This study is a single-center RCT that has been ongoing since March 1, 2021, at the Colorectal Cancer Center of Shanghai 10th People’s Hospital. Eligible patients will be enrolled and randomly assigned to receive either CBT or usual care (1:1). The overall study design is illustrated in Figure 1.
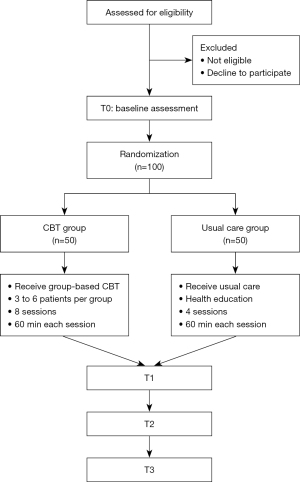
Study population
We plan to recruit 100 CRC patients who are undergoing chemotherapy.
Participants will be screened for inclusion if they meet the following criteria:
- Age ≥18 years and ≤75 years;
- Have an education of junior high school or higher;
- Be able to complete psychological condition assessment;
- Willing to participate and competent to provide informed consent;
- Have normal cognitive function at enrollment, indicates by a score greater than 23 on the Chinese version of the Mini-Mental State Examination (CMMSE) (30);
- Speak Chinese Mandarin as their native language;
- Have a CRC in stage IV going for first-line chemotherapy.
Participants who meet any of the following criteria will be excluded:
- History of malignancies;
- History of mental disorders (e.g., major depressive disorder and schizophrenia) according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V; American Psychiatric Association, 2013);
- History of brain trauma or neurologic diseases (e.g., epilepsy, stroke) according to the International Statistical Classification of Diseases and Related Health Problems, 11th Revision (ICD-11-version, 2022);
- Severe or untreated medical disorders (e.g., advanced cardiac or respiratory disease, severe liver, kidney, or metabolic disease);
- Receive adjuvant chemotherapy, neoadjuvant chemotherapy and immunotherapy;
- Alcohol dependency or drug abuse;
- Participation in other clinical studies.
Purpose of the study
The primary goal is to determine the effect of CBT on chemotherapy-induced side effects in CRC patients. We hypothesize that CRC patients who receive CBT will report fewer chemotherapy-induced side effects during chemotherapy.
The second goal is to determine the effect of CBT on the immune function of CRC patients during chemotherapy. We hypothesize that CRC patients who receive CBT will have lower levels of certain plasma inflammatory cytokines during chemotherapy.
Other goals are to examine (I) the effect of CBT on the level of tumor markers; (II) the effect of CBT on psychological status (including perception of stress, depression and anxiety, self-efficacy, quality of life, and sleep quality); and (III) other laboratory examinations (e.g., biochemical index and blood cell counts).
Intervention group: CBT group
The intervention will be performed in the ward’s post-operative recovery room each time the patient comes in for chemotherapy. Patients randomly assigned to the CBT group will receive usual oncology care (including nutrition assessment, stoma care, observation of chemotherapy drug adverse reactions, etc.) in addition to 8 sessions of group-based intervention (3–6 participants per group). Group is open because patients have different chemotherapy regimens and it is difficult for patients in the same group to be hospitalized for chemotherapy at the same time every time. Specifically, the CBT manual incorporates education, relaxation training, self-awareness of stress and how to deal with it, encouragement of stress expression, development of self-confidence, self-regulation training, identification of maladaptive coping, and encouragement of adaptive coping. Every intervention section will last for 60 minutes (45 minutes for CBT and 15 minutes for relaxation training) and will be conducted every 2–3 weeks within the period of each chemotherapy course.
Control group: usual care group
Patients randomly assigned to the usual care group (usual oncology care) will receive four sessions of health education monthly. Each session will last for 60 minutes, including lectures and question-and-answer segments. These four sessions will focus on (I) diagnosis and treatment of CRC; (II) adverse reactions and management of chemotherapy; (III) nutritional support; and (IV) physical exercise during chemotherapy.
Training
Two well-trained psychotherapists will conduct the CBT. They will receive professional training before the start of this study and will be regularly supervised by a senior clinical psychologist throughout the study.
Data collection
The data will be collected by trained research assistants and recorded on case report forms. The data will be stored in a secure database, and patients will be numerically coded to anonymize data. The data to be collected include the following.
Population characteristics
Demographic characteristics such as age, sex, body mass index (BMI), years of education, marital status, annual income, smoking, alcohol consumption, exercise, hobbies, Charlson comorbidity index (CCI), tumor grade, and number of chemotherapy courses will be collected at enrollment.
Baseline assessment (T0)
Clinical baseline data will be collected at enrollment approximately one week before chemotherapy.
Chemotherapy-induced side effects
Chemotherapy-induced side effects will be evaluated using the M.D. Anderson Symptom Inventory-Gastrointestinal Cancer Module (MDASI-GI), which includes 13 core symptom items, five gastrointestinal cancer-specific symptom items, and six interference items (31). The Chinese version of the MDASI-GI has demonstrated good reliability and validity among the Chinese population (32). The MDASI-GI will be assessed on the last day of each chemotherapy cycle.
Immune function
Inflammatory cytokines: C-reactive protein (CRP), IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ).
Other outcomes
(I) Psychological condition
Perception of stress. Perception of stress will be assessed using the 10-item Perceived Stress Scale (PSS-10), one of the most widely used psychological instruments, developed by Cohen et al. in 1983 (33). The scale involves 10 items scored from 0 to 4 with a total possible score of 40. Higher scores indicate greater perceived stress. The 10-item version has been confirmed to have good reliability and validity in the Chinese population (34).
Depression. Depression will be assessed using the Patient Health Questionnaire-9 (PHQ-9). It involves nine items scored from 0 to 3 with a total possible score of 27. The total score of the PHQ-9 ranges from 0 to 27 points, and the severity of depression increases as the total score increases. The scale has well-established reliability and validity in the Chinese population (35).
Anxiety. Anxiety will be assessed using the Generalized Anxiety Disorder-7 (GAD-7). It involves seven items scored from 0 to 3, with a total possible score of 21. The total score of the GAD-7 ranges from 0 to 21 points, and the severity of anxiety increases as the total score increases. The reliability and validity of this scale has been well established in the Chinese population (36).
Self-efficacy. The level of self-efficacy will be assessed using the General Self Efficacy Scale (GSES). The GSES was published in 1981 by Ralf Schwarzer and translated into Chinese in 1995 (37). It involves 10 items scored from 10 to 40. The final score is the sum of all items divided by 10, rated 1–4. Higher scores indicate greater self-efficacy. The Chinese version if the GSES has good reliability and validity (38).
Social support. Social support conditions will be assessed using the Social Support Rating Scale (SSRS). The scale was developed by Chinese scholar Shuiyuan Xiao in 1987 (39). It contains 10 items across three dimensions: objective support, subjective support, and utilization of social support. The total score is the sum of the 10 items. A higher score indicates greater social support. The scale has good reliability and validity in the Chinese population (40).
Quality of life. Health-related quality of life (HRQoL) will be measured using the European Organization for Research and Treatment of Cancer (EORTC), Quality of Life Questionnaire, Core 30 (QLQ-C30), and EORTC diagnosis-specific modules for colorectal cancer (QLQ-CR29). The two scales have been shown to have satisfactory reliability and validity (41,42).
Sleep quality. Sleep quality will be assessed using the Chinese version of the Pittsburgh Sleep Quality Index (PSQI) (40). It is a 9-item self-rating scale with a total summed score ranging from 0 to 21. Higher scores indicate lower sleep quality. The scale has good reliability and validity (43).
(II) Tumor markers
The following tumor markers will be measured: alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 125 (CA125), carbohydrate antigen 72-4 (CA 72-4), and carbohydrate antigen 242 (CA242).
(III) Laboratory examinations
- The following biochemical indices will be measured: liver and renal function, blood glucose, and lipid profile.
- Blood cell counts will be assessed.
Follow-up assessments
Time points: immediately post-intervention (T1), 3 months post-intervention (T2), and 6 months post-intervention (T3).
Contents: all baseline measures except SSRS.
Sample size estimation
Sample size calculation was based on the results of a previous RCT conducted among breast cancer patients to determine the effect of cognitive therapy on immune function (44). For the current study, we determined the effect size of Cohen’s d=0.67 for the group difference in the change in interleukin-1 (IL-1) between the intervention group and control group. With a type I error of 5% and a power of 90%, we needed a total of 72 patients (36 per group). Anticipating a dropout rate of approximately 20%, we plan to enroll a total of 100 patients (50 per group) to ensure that we can include 72 participants in the final analysis.
Randomization and blinding
A researcher who is independent of the study will be responsible for randomization and allocation of participants at the beginning of the study. Participants will be randomly assigned to either a CBT group or a usual care group at a 1:1 ratio.
The therapists performing the intervention cannot be blinded to the allocation because they will need to use the psychotherapy manual during intervention. Another research assistant who is blinded to the allocations will perform psychological assessments and conduct clinical examinations of the participants.
Statistical analysis
Descriptive data will be reported as the mean ± SD (standard deviation) for continuous variables and counts or percentages for categorical variables. The Kolmogorov-Smirnov test will be used to test the normality of all variables. An independent t-test and a chi-squared test will be used to compare general characteristics, psychological conditions, and immune function between the two groups. The changes in psychological conditions and immune function at different time points will be examined using linear mixed models.
Statistical analysis of the data will be performed using SPSS version 26.0 (IBM, Armonk, New York, USA). A two-sided P value less than 0.05 was considered statistically significant for all analyses.
Patient and public involvement
Neither patients nor the public will be involved in identifying the research question or the design of the study. The results of the study will be disseminated to the public at the completion of the trial.
Data and safety monitoring board and interim analysis
Electronic data will be securely stored, while all remaining data will be documented on paper case report forms and later transcribed into electronic format. Regular monitoring of the data will be conducted, and the principal investigators or their representatives will perform interim data analyses to ensure data quality, such as identifying and excluding any data errors resulting from technical issues.
Handling of missing data
The respective analyses will involve the imputation of missing data. In the case of data missing completely at random, the expectation-maximization (EM) algorithm will be utilized for replacement. However, if the missing data is not completely at random, multiple imputation will be employed.
Trial status
The trial has been ongoing since March 1, 2021. We expect data collection to be completed by December 31, 2023.
Ethics and dissemination
Written informed consent documents will be obtained from all the participants. Participants will be informed of the purpose of the study, the voluntary nature, the risks and benefits, and the right to refuse or withdraw from the study. CBT is an intervention that will not cause any physical or psychological harm to the participants. However, if any other unanticipated problem is reported or the participants feel discomfort during the assessment, participants may choose not to answer any questions or terminate the assessment. Participants will be asked to sign two copies of the consent form, one of which will be kept by the participants, and the other will be retained by the researcher. All information collected without personal identifiers will be stored safely in a locked file cabinet in the principal investigator’s office. Electronic data will be kept in a computer secured with a password. The principal investigator of the study will have access to the final trial dataset. The results will be published in peer-reviewed journals. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Shanghai 10th People’s Hospital (No. SHSY-IEC-4.1/20-268/01). In addition, the principal investigator will submit research progress reports to the ethics committee regularly.
Discussion
This is a single-center RCT. In this study, we will conduct CBT for CRC patients undergoing chemotherapy. CRC patients face a higher incidence of depression and anxiety during chemotherapy treatment (45). In addition, one study showed that after controlling for cancer characteristics, every one-standard deviation (1-SD) increase in anxiety or depression symptoms was associated with 17% and 20% increased in the risk of mortality, respectively (18), indicating that anxiety and depression are significantly related to elevated mortality risk in CRC patients. Other studies have demonstrated that the degree of chemotherapy-induced side effects is affected by multiple factors, including depression and anxiety (46-48).
Many tumor treatment guidelines advocate for the inclusion of psychosocial intervention as a crucial component of therapy throughout the entirety of tumor treatment (49-52). Cognitive Behavioral Therapy (CBT) emerges as a significant psychological treatment modality that offers noninvasiveness, convenience, and safety for patients with colorectal cancer (CRC). Substantial evidence supports the efficacy of CBT in significantly ameliorating anxiety and depression among individuals diagnosed with cancer (53,54). Moreover, some studies have examined the positive impact of CBT on fatigue reduction in cancer patients (24,55). Therefore, CBT maybe a promising method that has the potential to reduce the side effects of chemotherapy. In addition, CBT has been shown to improve immune function in other patients, such as human immunodeficiency virus (HIV) and sleep disturbance (56,57), but there have been few studies in CRC patients.
In this study, we will focus on the effect of CBT and determine the differences in chemotherapy-induced side effects and immune function between CRC patients who receive CBT and those who do not receive CBT. We expect that the outcomes of this RCT will provide clinical evidence regarding whether CBT should be generalized in clinical treatment and the extent to which CBT reduces chemotherapy-induced side effects for CRC patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-625/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-625/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-625/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Shanghai 10th People’s Hospital (No. SHSY-IEC-4.1/20-268/01) and informed consent will be taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Islami F, Chen W, Yu XQ, et al. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol 2017;28:2567-74. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022;7:627-47. [Crossref] [PubMed]
- Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. [Crossref] [PubMed]
- Lu LC, Tsay SL, Chang SY, et al. Daily activity, mood, and quality of life in colorectal cancer patients with chemotherapy-induced peripheral neuropathy: A mediation effect analysis. Cancer Med 2019;8:963-71. [Crossref] [PubMed]
- Révész D, Bonhof CS, Bours MJL, et al. Sociodemographic, Clinical, Lifestyle, and Psychological Correlates of Peripheral Neuropathy among 2- to 12-Year Colorectal Cancer Survivors. Oncol Res Treat 2022;45:480-93. [Crossref] [PubMed]
- Trompetter HR, Bonhof CS, van de Poll-Franse LV, et al. Exploring the relationship among dispositional optimism, health-related quality of life, and CIPN severity among colorectal cancer patients with chronic peripheral neuropathy. Support Care Cancer 2022;30:95-104. [Crossref] [PubMed]
- El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin 2015;65:428-55. [Crossref] [PubMed]
- Mols F, Schoormans D, de Hingh I, et al. Symptoms of anxiety and depression among colorectal cancer survivors from the population-based, longitudinal PROFILES Registry: Prevalence, predictors, and impact on quality of life. Cancer 2018;124:2621-8. [Crossref] [PubMed]
- Aminisani N, Nikbakht H, Asghari Jafarabadi M, et al. Depression, anxiety, and health related quality of life among colorectal cancer survivors. J Gastrointest Oncol 2017;8:81-8. [Crossref] [PubMed]
- Wu HJ, Chuang CM, Chien CH, et al. Changes in Depression and Sleep Quality and Associated Factors in Women Receiving Chemotherapy for Ovarian Cancer: An Observational Study. Cancer Nurs 2022;45:271-9. [Crossref] [PubMed]
- Lim CC, Devi MK, Ang E. Anxiety in women with breast cancer undergoing treatment: a systematic review. Int J Evid Based Healthc 2011;9:215-35. [PubMed]
- Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014;1:343-50. [Crossref] [PubMed]
- Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev 2018;4:CD011006. [Crossref] [PubMed]
- Trudel-Fitzgerald C, Tworoger SS, Zhang X, et al. Anxiety, Depression, and Colorectal Cancer Survival: Results from Two Prospective Cohorts. J Clin Med 2020;9:3174. [Crossref] [PubMed]
- Trudel-Fitzgerald C, Tworoger SS, Poole EM, et al. Psychological symptoms and subsequent healthy lifestyle after a colorectal cancer diagnosis. Health Psychol 2018;37:207-17. [Crossref] [PubMed]
- Lloyd S, Baraghoshi D, Tao R, et al. Mental Health Disorders are More Common in Colorectal Cancer Survivors and Associated With Decreased Overall Survival. Am J Clin Oncol 2019;42:355-62. [Crossref] [PubMed]
- Zhu J, Fang F, Sjölander A, et al. First-onset mental disorders after cancer diagnosis and cancer-specific mortality: a nationwide cohort study. Ann Oncol 2017;28:1964-9. [Crossref] [PubMed]
- McNeish BL, Richardson JK, Whitney DG. Chemotherapy-induced peripheral neuropathy onset is associated with early risk of depression and anxiety in breast cancer survivors. Eur J Cancer Care (Engl) 2022;31:e13648. [Crossref] [PubMed]
- Bonhof CS, van de Poll-Franse LV, Vissers PAJ, et al. Anxiety and depression mediate the association between chemotherapy-induced peripheral neuropathy and fatigue: Results from the population-based PROFILES registry. Psychooncology 2019;28:1926-33. [Crossref] [PubMed]
- Sutanto YS, Ibrahim D, Septiawan D, et al. Effect of Cognitive Behavioral Therapy on Improving Anxiety, Depression, and Quality of Life in Pre-Diagnosed Lung Cancer Patients. Asian Pac J Cancer Prev 2021;22:3455-60. [Crossref] [PubMed]
- Landa-Ramírez E, Greer JA, Sánchez-Román S, et al. Tailoring Cognitive Behavioral Therapy for Depression and Anxiety Symptoms in Mexican Terminal Cancer Patients: A Multiple Baseline Study. J Clin Psychol Med Settings 2020;27:54-67. [Crossref] [PubMed]
- Baussard L, Cousson-Gélie F, Jarlier M, et al. Hypnosis and cognitive behavioral therapy with online sessions to reduce fatigue in patients undergoing chemotherapy for a metastatic colorectal cancer: Rational and study protocol for a feasibility study. Front Psychol 2022;13:953711. [Crossref] [PubMed]
- Green McDonald P, O’Connell M, Lutgendorf SK. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun 2013;30:S1-9. [Crossref] [PubMed]
- Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun 2010;24:1-8. [Crossref] [PubMed]
- Kakoo Brioso E, Ferreira Cristina S, Costa L, et al. Correlation between emotional regulation and peripheral lymphocyte counts in colorectal cancer patients. PeerJ 2020;8:e9475. [Crossref] [PubMed]
- Oliveira Miranda D, Soares de Lima TA, Ribeiro Azevedo L, et al. Proinflammatory cytokines correlate with depression and anxiety in colorectal cancer patients. Biomed Res Int 2014;2014:739650. [Crossref] [PubMed]
- McGregor BA, Antoni MH, Boyers A, et al. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res 2004;56:1-8. [Crossref] [PubMed]
- Li H, Jia J, Yang Z. Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J Alzheimers Dis 2016;53:487-96. [Crossref] [PubMed]
- Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000;89:1634-46. [Crossref] [PubMed]
- Chen RW. Validation and application of the Chinese version of the MD Anderson symptom inventory - Gastrointestinal Cancer Module (MDASI-GI-C). Wuhan: Huazhong University of Science and Technology; 2019.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385-96. [Crossref] [PubMed]
- Wang Z, Wang Y, Wu ZG, et al. Reliability and validity of the Chinese version of Perceived Stress Scale. Journal of Shanghai Jiao Tong University 2015;35:1448-51. (Medical Science).
- Wang W, Bian Q, Zhao Y, et al. Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry 2014;36:539-44. [Crossref] [PubMed]
- He XY, Li CB, Qian J, et al. Reliability and validity of a generalized anxiety disorder scale in general hospital outpatients. Shanghai Archives of Psychiatry 2010;22:200-3.
- Zhang JX, Schwarzer R. Measuring optimistic self-beliefs: A Chinese adaptation of the general self-efficacy scale. Psychologia 1995;38:174-81.
- Cheung SK, Sun SY. Assessment of optimistic self-beliefs: further validation of the Chinese version of the General Self-Efficacy Scale. Psychol Rep 1999;85:1221-4. [Crossref] [PubMed]
- Xiao SY, Yang DS. The impact of social support on physical and mental health. Chinese Mental Health Journal 1987;1:183-7.
- Xiao S. The theoretical basis and research application of Social Support Rating Scale. Journal of Clinical Psychiatry 1994;4:98-100.
- Lin JB, Zhang L, Wu DW, et al. Validation of the chinese version of the EORTC QLQ-CR29 in patients with colorectal cancer. World J Gastroenterol 2017;23:1891-8. [Crossref] [PubMed]
- Wan C, Meng Q, Yang Z, et al. Validation of the simplified Chinese version of EORTC QLQ-C30 from the measurements of five types of inpatients with cancer. Ann Oncol 2008;19:2053-60. [Crossref] [PubMed]
- Liu XC, Tang MQ, Hu L, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry 1996;29:103-7.
- Savard J, Simard S, Giguère I, et al. Randomized clinical trial on cognitive therapy for depression in women with metastatic breast cancer: psychological and immunological effects. Palliat Support Care 2006;4:219-37. [Crossref] [PubMed]
- Burgers K, Moore C, Bednash L. Care of the Colorectal Cancer Survivor. Am Fam Physician 2018;97:331-6. [PubMed]
- Kleckner IR, Jusko TA, Culakova E, et al. Longitudinal study of inflammatory, behavioral, clinical, and psychosocial risk factors for chemotherapy-induced peripheral neuropathy. Breast Cancer Res Treat 2021;189:521-32. [Crossref] [PubMed]
- Lee KM, Jung D, Hwang H, et al. Pre-treatment anxiety is associated with persistent chemotherapy-induced peripheral neuropathy in women treated with neoadjuvant chemotherapy for breast cancer. J Psychosom Res 2018;108:14-9. [Crossref] [PubMed]
- Zhou X, Wang DY, Ding CY, et al. Psychosocial adaptation and influencing factors among patients with chemotherapy-induced peripheral neuropathy. World J Clin Cases 2022;10:4843-55. [Crossref] [PubMed]
- Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 2017;67:194-232. [Crossref] [PubMed]
- Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol 2014;32:1605-19. [Crossref] [PubMed]
- Caminiti C, Diodati F, Annunziata MA, et al. Psychosocial Care for Adult Cancer Patients: Guidelines of the Italian Medical Oncology Association. Cancers (Basel) 2021;13:4878. [Crossref] [PubMed]
- Osman H, Shrestha S, Temin S, et al. Palliative Care in the Global Setting: ASCO Resource-Stratified Practice Guideline. J Glob Oncol 2018;4:1-24. [PubMed]
- Zhang L, Liu X, Tong F, et al. Cognitive behavioral therapy for anxiety and depression in cancer survivors: a meta-analysis. Sci Rep 2022;12:21466. [Crossref] [PubMed]
- Sun H, Huang H, Ji S, et al. The Efficacy of Cognitive Behavioral Therapy to Treat Depression and Anxiety and Improve Quality of Life Among Early-Stage Breast Cancer Patients. Integr Cancer Ther 2019;18:1534735419829573. [Crossref] [PubMed]
- Poort H, Peters MEWJ, van der Graaf WTA, et al. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann Oncol 2020;31:115-22. [Crossref] [PubMed]
- Crepaz N, Passin WF, Herbst JH, et al. Meta-analysis of cognitive-behavioral interventions on HIV-positive persons’ mental health and immune functioning. Health Psychol 2008;27:4-14. [Crossref] [PubMed]
- Chen HY, Cheng IC, Pan YJ, et al. Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int 2011;80:415-22. [Crossref] [PubMed]


