
The prognostic role of gastrointestinal bleeding in patients with a primary gastrointestinal stromal tumor: a long-term follow-up study
Highlight box
Key findings
• Although gastrointestinal (GI) bleeding occurred more frequently in small intestine, it was not identified as an independent prognostic factor in primary GI stromal tumor (GIST) who underwent radical resection.
What is known and what is new?
• Tumor size, tumor location, tumor rupture, and mitotic index (MI) are typical prognostic factors in primary GIST. Some studies had reported the prognostic role of GI bleeding, but the conclusions were inconsistent.
• The meta analysis including the results of present study and 6 previously reported studies showed that GI bleeding was not associated with the long-term prognosis of primary GIST.
What is the implication, and what should change now?
• The association between GI bleeding and prognosis remain to be further investigated. Future studies are required to define whether GI bleeding should be identified as a special form of tumor rupture.
Introduction
Gastrointestinal stromal tumors (GISTs) account for about 20% of soft tissue sarcomas and are considered the most common mesenchymal tumor in the digestive tract (1). GISTs can occur at any site in the digestive tract; the most common site is the stomach (50–60%), followed by the small intestine (30–35%), and the colorectum (5%). Only a few tumors (<5%) occur outside the gastrointestinal (GI) tract [extra-GIST (E-GIST)] (2). Although surgical resection is considered the essential treatment (3), many patients experience tumor recurrence (4). In previous studies, patients with a GIST who underwent surgical resection have displayed a recurrence-free survival (RFS) rate of 30–50% at 5 years without adjuvant imatinib therapy (5,6). Mitotic index (MI), tumor rupture, tumor size, and tumor location are typical prognostic factors (7). The most widely used risk stratification for primary GIST after radical resection is the modified National Institutes of Health (NIH) stratification (year 2008), which is based on different combinations of the above four factors (8). However, some researchers have demonstrated that the modified NIH stratification (year 2008) may have some defects. Several methods for a recurrence risk assessment have been proposed, but the clinical utility needs further investigation (9,10).
Tumor rupture was suggested for inclusion in the NIH high-risk category and was considered to confer increased recurrence risk to patient prognosis after surgical resection (11). Meanwhile, GI bleeding has been considered to indicate a “rupture in the gastrointestinal tract”, and its prognostic impact on a primary GIST after complete resection has become a concern among many researchers. GI bleeding, abdominal pain, abdominal mass, anemia, nausea, and vomiting are the common clinical symptoms of GISTs (1). These symptoms are generally believed to be related to the tumor location, tumor size, and growth pattern of the tumor. According to reports, about 20–50% of patients with a GIST present with GI bleeding, including melena, hematochezia, and hematemesis (12,13). In recent years, several studies have reported the prognostic role of GI bleeding for GIST patients. Wan et al. (14) concluded that the patients with GI bleeding had a superior RFS and overall survival (OS) compared with those without GI bleeding. However, Pih et al. (15) showed that GI bleeding was associated with worse 5-year survival rates of gastric GIST. Therefore, the association between GI bleeding and long-term prognosis of GIST patients has remained unclear. Herein, we conducted a retrospective study and performed a meta-analysis to investigate the clinical and prognostic features of GIST patients with GI bleeding. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1240/rc).
Methods
Patients
A retrospective analysis was performed to assess the prognostic role of GI bleeding in patients with a GIST. Patients who had undergone complete resection of a primary GIST in our hospital from January 2003 to December 2008 were retrospectively reviewed. Clinical data including age, sex, clinical symptoms, pathological reports, and peripheral blood tests were obtained from medical records. The histopathological evaluation and immunohistochemistry of cluster of differentiation 117 (CD117) were used to diagnose the GIST. No sample size calculation was performed due to the retrospective nature of this study (16). The major inclusion criteria were as follows: (I) patients with GI bleeding symptoms; (II) R0 resection for primary GISTs; (III) tumor size >2.0 cm; (IV) an Eastern Cooperative Oncology Group (ECOG) performance status score of 2 or less; (V) survival of more than 1 month after surgery. The exclusion criteria were as follows: (I) tumor size ≤2.0 cm; (II) E-GIST; (III) distant metastasis already present when the tumor was diagnosed; (IV) intraoperative tumor residual (R1 or R2 resection); (V) preoperative or postoperative chemotherapy, radiotherapy, or targeted therapy (including neoadjuvant or adjuvant imatinib therapy); (VI) patients without GI bleeding symptoms but a positive fecal occult blood test; (VII) other malignant tumors; (VIII) missing data and incomplete variables. Figure 1 displays the patient selection flowchart. This study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University [No. 2019(1360)]. The patients’ identities were kept anonymous, and the requirement for informed consent was waived and omitted for this retrospective analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).

GI bleeding was diagnosed by the presence of melena, hematochezia, or hematemesis, with or without anemia, but a positive fecal occult blood test without GI bleeding symptoms was excluded. According to the definition, patients were divided into two different groups: patients who presented with GI bleeding (GB group), and the rest who did not present with GI bleeding (NGB group).
Follow-up
Follow-up was performed routinely every 3–6 months for the first 3 years, then every 6–9 months until 5 years, and every year after 5 years. Peripheral blood tests, abdominal computed tomography (CT), and chest X-rays were routinely performed during the follow-up. Endoscopy, biopsy, or other imaging examinations were performed if necessary. Follow-up information was obtained from out-patient medical records, the hospital tumor registry, or direct contact with patients or their families. The last follow-up was performed in May 2019.
Systematic review and meta-analysis
Literature search and selection
A comprehensive and systematic search of the PubMed, Embase, Cochrane Collaboration, and Medline databases was undertaken to identify relevant studies published between 2000 and 2022. The terms relating to “gastrointestinal stromal tumor”, “gastrointestinal stromal tumour”, and “GIST” were combined with terms relating to “bleeding” and “gastrointestinal bleeding”. The following Medical Subject Headings (MeSH) were used: “Gastrointestinal stromal tumors”, “Gastrointestinal Hemorrhage”, “Hematemesis”, and “Melena”, combined with “Disease-Free Survival”, “Recurrence”, “Neoplasm Recurrence, Local”, and “Survival Analysis”. An additional search was conducted on the references of studies that met the inclusion criteria for other trials or reports that were relevant to this meta-analysis.
The inclusion criteria were as follows: (I) the participants of included studies were diagnosed with primary GIST based on histology and immunohistochemistry; (II) the exposure of interest was GI bleeding; (III) the outcome of interest was RFS; (IV) odds ratio (OR), relative risk (RR), or hazard ratio (HR) with a corresponding 95% confidence interval (CI) are provided; (V) retrospective or prospective cohort study. No publication language restriction was applied in the present meta-analysis (Figure S1).
Data extraction and quality assessment
Data extraction was carried out independently by two authors. The following information was extracted from each publication: author, publication year, country, study design, age of participants, date of diagnosis and treatment, follow-up period, GI-bleeding/non-GI-bleeding, and OR/RR/HR for RFS. The most adequately adjusted model was selected to evaluate the risk value for the final analysis. Differences in data extraction between investigators were resolved by consensus.
The Newcastle-Ottawa scale (NOS) was used to assess the quality of the included studies, which involves 3 main quality parameters: 4 items for selection, 2 items for comparability, and 3 items for outcome assessment (17). The score of each study ranged from 0 to 9 stars, with a score of ≥7 being considered a high-quality study (Table S1).
Statistical analysis
Categorical variables were analyzed with the chi-square test. Continuous variables were represented by median values with range. One-to-one propensity score matching (PSM) was used to control the unbalanced distributions of covariates between the GB and NGB groups. Finally, age, gender, mitotic rate, tumor size, tumor location, tumor rupture, and the risk stratification were included in PSM. PSM was conducted based on the logistic regression of the propensity score, and the caliper was 0.02. RFS was defined as the time from surgery to the tumor recurrence, metastasis, or death from any cause, whichever happened first. Patients who were alive and free of tumor recurrence/metastasis in May 2019 were censored from the RFS analysis. RFS was conducted by the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazard model was used for the multivariate analysis to determine the independent prognosis factors, and HR with 95% CI was also calculated. The software SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses. A 2-sided P value <0.05 was considered significant.
The software Stata 12.0 (StataCorp., LLC, College Station, TX, USA) was used to conduct the systematic review. The HR and 95% CI from each included study to compare the prognosis between the two groups were collected. The potential heterogeneity between the included studies was assessed using the Q tests and I2 statistics. In the analysis, P<0.10 or I2>50% was considered to indicate substantial heterogeneity, and the random-effects model was used; otherwise, the fixed-effects model was used. The sensitivity analysis was evaluated using the remove a single study approach, and then we recalculated the results.
Results
Clinical and pathological features
A total of 218 patients with primary GIST (>2.0 cm) who received surgery from January 2003 to December 2008 in our hospital were retrospectively reviewed. According to the inclusion and exclusion criteria, 84 patients with GI bleeding and 90 patients without GI bleeding were finally included in this study (Figure 1). Among the 84 patients, there were 44 (52.4%) males and 40 (47.6%) females. The median age was 57 years (range, 20–89 years). The GISTs had grown in the stomach in 27 (32.1%) patients, duodenum in 14 (16.7%), small intestine in 41 (48.8%), and the colorectum in 2 (2.4%) patients. The median size of the tumor was 5.0 cm (range, 2.1–13.0 cm), and the median MI was 2 (range, 0–100) per 50 high power fields. With the modified NIH risk stratification (year 2008), 51 (60.7%) patients were classified as low risk, 9 (10.7%) as intermediate risk, and 24 (28.6%) as high risk. Compared to the 90 patients without GI bleeding, the GIST-related GI bleeding occurred more frequently in the small intestine (Table 1). Each of the 2 groups included 45 patients after the PSM, and all the covariables were comparable (P>0.05, Table 1).
Table 1
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| GB group (N=84) | NGB group (N=90) | P* | GB group (N=45) | NGB group (N=45) | P* | ||
| Gender | 0.570 | 0.673 | |||||
| Male | 44 | 51 | 24 | 22 | |||
| Female | 40 | 39 | 21 | 23 | |||
| Age (years) | 0.362 | 0.667 | |||||
| ≤60 | 56 | 54 | 28 | 26 | |||
| >60 | 28 | 36 | 17 | 19 | |||
| Tumor size (cm) | 0.071 | 0.734 | |||||
| 2.1–5.0 | 54 | 51 | 24 | 24 | |||
| 5.1–10.0 | 27 | 27 | 18 | 16 | |||
| >10.0 | 3 | 12 | 3 | 5 | |||
| MI (/50 HPFs) | 0.601 | 0.842 | |||||
| 0–5 | 79 | 81 | 42 | 43 | |||
| 6–10 | 3 | 6 | 2 | 1 | |||
| >10 | 2 | 3 | 1 | 1 | |||
| Tumor location | <0.001 | 0.324 | |||||
| Stomach | 27 | 62 | 26 | 29 | |||
| Duodenum | 14 | 3 | 1 | 3 | |||
| Small Intestine | 41 | 16 | 16 | 13 | |||
| Colorectum | 2 | 9 | 2 | 0 | |||
| Tumor rupture | 0.115 | 1.000 | |||||
| Yes | 1 | 5 | 1 | 1 | |||
| No | 83 | 85 | 44 | 44 | |||
| Recurrence risk | 0.763 | 1.000 | |||||
| Low | 51 | 50 | 23 | 23 | |||
| Intermediate | 9 | 12 | 9 | 9 | |||
| High | 24 | 28 | 13 | 13 | |||
*, the Chi-square test was used. PSM, propensity score matching; GB, gastrointestinal bleeding; NGB, non-gastrointestinal bleeding; MI, mitotic index; HPFs, high-powered fields.
Survival analysis
The median time of follow-up was 140 months (range, 10–196 months) of all patients, and 38 patients developed tumor recurrence/metastasis. The most common site of distant metastasis was the liver (16 patients), followed by the peritoneum (10 patients). Of all patients in the study, at the time of the most recent follow-up, 127 patients were alive without disease, 12 were alive with tumor recurrence, 26 had died from tumor recurrence, and 9 died from other causes. The univariate analysis showed that MI (HR =7.11, 95% CI: 3.58–14.15, P<0.001), tumor location (HR =3.42, 95% CI: 1.77–6.59, P<0.001), tumor size (HR =2.63, 95% CI: 1.47–4.72, P=0.001), and age (HR =2.15, 95% CI: 1.21–3.82, P=0.009) were associated with RFS (Table 2). However, GI bleeding was not associated with RFS (HR =1.21, 95% CI: 0.68–2.14, P=0.518, Figure 2A). The multivariate analysis showed that only tumor location (HR =3.48, 95% CI: 1.78–6.82, P<0.001), tumor size (HR =1.91, 95% CI: 1.05–3.47, P=0.035), MI (HR =5.69, 95% CI: 2.77–11.67, P<0.001), and age (HR =2.68, 95% CI: 1.49–4.82, P=0.001) were independent prognostic factors (Table 2). After PSM, the GI bleeding was still not associated with RFS (HR =1.23, 95% CI: 0.51–2.97, P=0.642, Figure 2B). In the GI bleeding group, 73 patients had preoperative hemoglobin (Hb) values available: 4 patients (5.5%) had an Hb level of 30–60 g/L, 28 patients (38.4%) had 60–90 g/L, 23 (31.5%) patients had 90–120 g/L, and 18 patients (24.7%) had a level of >120 g/L. The univariate analysis showed that the different Hb levels were not associated with RFS in the GB group (P=0.928) (Figure 3).
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Gender | |||||||
| Male | Ref | ||||||
| Female | 0.65 | 0.36–1.17 | 0.149 | ||||
| Age (years) | |||||||
| ≤60 | Ref | Ref | |||||
| >60 | 2.15 | 1.21–3.82 | 0.009 | 2.68 | 1.49–4.82 | 0.001 | |
| Tumor size (cm) | |||||||
| 2.1–5.0 | Ref | Ref | |||||
| >5.0 | 2.63 | 1.47–4.72 | 0.001 | 1.91 | 1.05–3.47 | 0.035 | |
| MI (/50 HPFs) | |||||||
| 0–5 | Ref | Ref | |||||
| >5 | 7.11 | 3.58–14.15 | <0.001 | 5.69 | 2.77–11.67 | <0.001 | |
| Tumor location | |||||||
| Stomach | Ref | Ref | |||||
| Non-stomach | 3.42 | 1.77–6.59 | <0.001 | 3.48 | 1.78–6.82 | <0.001 | |
| Tumor rupture | |||||||
| No | Ref | ||||||
| Yes | 2.62 | 0.81–8.47 | 0.107 | ||||
| GI bleeding | |||||||
| No | Ref | ||||||
| Yes | 1.21 | 0.68–2.14 | 0.518 | ||||
RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; Ref, reference; MI, mitotic index; HPFs, high-powered fields; GI, gastrointestinal.

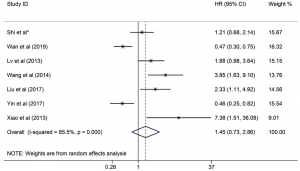
Pooled results for the prognostic role of GI bleeding in GIST patients with meta-analysis
Finally, 7 studies [the present study and 6 published studies (14,18-22)] fulfilled the inclusion criteria (Table 3). In the equality assessment, 4 studies scored 7–8, and were therefore considered of high-quality; 2 studies scored 6 stars. Of the 1,889 patients included in the meta-analysis, 621 (32.9%) patients presented with GI bleeding, whereas the other 1,268 (67.1%) patients did not present GI bleeding. The potential heterogeneity between the included studies was statistically significant (I2=85.5%, P<0.001). Finally, the pooled HR was 1.45 (95% CI: 0.73–2.86, P=0.287; Figure 4), indicating that GI bleeding was not associated with RFS. The sensitivity analysis of the prognostic role of GI bleeding in patients with a primary GIST showed no change in the pooled statistical HR significance after the sequential removal of each study.
Table 3
| Study | No. of patients (GB/total) | 5-year RFS, % | HR (95% CI) |
|---|---|---|---|
| Shi et al.* | 84/174 | 86.90 | 1.21 (0.68–2.14) |
| Wan et al., 2019 (14) | 236/800 | NA | 0.47 (0.30–0.75) |
| Yin et al., 2017 (18) | 163/526 | 88.50 | 0.46 (0.25–0.82) |
| Xiao et al., 2013 (19) | 7/21 | 33.0 | 7.38 (1.51–36.08) |
| Liu et al., 2017 (20) | 63/170 | NA | 2.33 (1.11–4.92) |
| Lv et al., 2013 (21) | 44/114 | 50.70 | 1.88 (0.98–3.64) |
| Wang et al., 2014 (22) | 24/84 | 36.40 | 3.85 (1.63–9.10) |
*, the data in present study. GIST, gastrointestinal stromal tumor; GB, gastrointestinal bleeding; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; NA, not available.
Discussion
GIST is thought to originate from the Cajal cells of the GI tract (1), and GI bleeding is one of the most frequent symptoms in GISTs (2). To date, 2 mechanisms have been reported to result in GI bleeding: (I) the tumor could alter the blood supply of the local mucosa when it grows large. Subsequently, the mucosal barrier is damaged and ulcerative bleeding occurs (23); (II) the tumor could invade the mucosal or submucosal blood vessels, and eventually cause bleeding (24). Both of the mechanisms indicated that GISTs accompanied by GI bleeding may have higher malignant potential. Furthermore, it has been reported that tumor location (small intestine), small tumors (≤5.0 cm), prolonged prothrombin time, male gender, age (≤60 years), presence of surface dimpling on CT, and Ki-67 and S-100 positivity are deemed as an increased risk in GIST patients with GI bleeding (10,14). In this study, the proportion of GI bleeding was 48.3% and it occurred more frequently in the small intestine (including the duodenum), which was consistent with previous studies.
The growth pattern of primary GISTs in the GI tract includes endoluminal, exophytic, and mixed (dumbbell-shaped) (25). Of the 3 growth patterns, exophytic growth is the most frequent, and larger tumors often grow in this pattern and are more likely to rupture in the abdominal cavity (26). Tumor rupture is considered the most relevant factor for tumor recurrence of primary GISTs after surgery. Nishida et al. proposed 6 manifestations of tumor rupture (27): (I) tumor fracture or spillage; (II) blood-stained ascites; (III) GI perforation at the tumor site; (IV) microscopic infiltration of an adjacent organ; (V) intralesional dissection or piecemeal resection; (VI) incisional biopsy. However, whether GI bleeding indicates another special definition of tumor rupture is uncertain, and only a few studies have reported the influence of GI bleeding on the prognosis.
In 2013, Lv et al. first reported the prognostic role of GI bleeding in primary GISTs after radical surgery (21). Some 44 of 114 patients diagnosed with a primary GIST who underwent R0 resection presented with GI bleeding. Univariate and multivariate analyses showed that GI bleeding predicted a poor prognosis for primary GISTs, and they ascribed the poor prognosis to hypo-immunity and a low Karnofsky score caused by GI bleeding (21). Some studies have also asserted that GI bleeding is a significant factor in a poor prognosis, and should be identified as a special form of rupture (15,20,28). However, there are several different points of view. Wan et al. retrospectively reviewed 800 patients with a primary GIST, and conducted PSM analysis to reduce the confounders. The results showed that GIST patients presenting with GI bleeding had a better RFS, compared to those without GI bleeding (14). Another study also showed that patients with GI bleeding generally have smaller tumors and a longer RFS compared to those without GI bleeding (18). They speculated that GI bleeding renders patients more vigilant than others, which facilitates an earlier diagnosis and surgical treatment.
In the present study, we retrospectively reviewed patients before 2009. Imatinib was not widely used for the adjuvant treatment of GIST patients during this period, and only 13 patients were excluded. We considered that this study might provide a better reflection of the natural course of GIST patients after radical surgery and that the median follow-up time was long enough (>10 years). As the occult blood test may be confounded by many factors, only patients with apparent GI bleeding were included in this study. Finally, 84 patients with GI bleeding and 90 patients without GI bleeding were included. A total of 45 patients from each group were chosen according to the PSM analysis. The survival analysis of both before and after PSM showed that GI bleeding was not an independent prognostic factor, and this analysis finding was inconsistent with the previous reports. Further, the additional analysis revealed that different Hb values were not associated with RFS in GB patients.
Since there are different views on whether GI bleeding influences the prognosis of GIST patients or not, we conducted a meta-analysis on several published articles. Finally, 7 studies (the present study and 6 that had been previously published) comprising 1,889 patients were included. Of the 6 studies from the database, 4 studies reported poor prognosis, and 2 reported favorable prognosis. The pooled results of the 7 studies also supported our results. However, only Wang et al. excluded patients receiving imatinib treatment (22). The rest of the studies included patients who received imatinib treatment but did not specify the number of patients receiving imatinib in each group, which limits the additional analysis of the effect of imatinib on RFS.
This study had several limitations. At first, the sample size was relatively small due to the limited number of patients at the single center. Secondly, the evidence of KIT/PDGFRA mutations in most of the patients was absent or undetected, because the retrospective study we conducted involved a review of GIST patients before 2009. Consequently, the associations between the mutations and prognosis could not be evaluated. Thus, further studies involving more patients from multiple institutions are needed to verify the results.
Conclusions
In conclusion, tumor location, tumor size, MI, and age were found to be independent prognostic factors associated with RFS in patients who underwent the radical operation of a primary GIST. Although GI bleeding in the small intestine occurred more frequently than in any other area, it was not identified as an independent prognostic factor in this study. Further studies are required to define whether GI bleeding should be identified as a special form of tumor rupture.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1240/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1240/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1240/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1240/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University [No. 2019(1360)] and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blay JY, Kang YK, Nishida T, et al. Gastrointestinal stromal tumours. Nat Rev Dis Primers 2021;7:22. [Crossref] [PubMed]
- von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol 2018;36:136-43. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Yang W, Shou C, Chen Z, et al. Reassessment of the recurrence risk of primary gastrointestinal stromal tumour after complete resection. Scand J Gastroenterol 2023;58:684-92. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg 2006;244:176-84. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Li J, Ye Y, Wang J, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res 2017;29:281-93. [Crossref] [PubMed]
- Wu H, Ding P, Wu J, et al. A New Online Dynamic Nomogram: Construction and Validation of a Predictive Model for Distant Metastasis Risk and Prognosis in Patients with Gastrointestinal Stromal Tumors. J Gastrointest Surg 2023;27:1429-44. [Crossref] [PubMed]
- Guan SH, Wang Q, Ma XM, et al. Development of an innovative nomogram of risk factors to predict postoperative recurrence of gastrointestinal stromal tumors. World J Gastrointest Surg 2022;14:940-9. [Crossref] [PubMed]
- Hølmebakk T, Bjerkehagen B, Hompland I, et al. Relationship between R1 resection, tumour rupture and recurrence in resected gastrointestinal stromal tumour. Br J Surg 2019;106:419-26. [Crossref] [PubMed]
- Wang L, Ni Z, Xu W, et al. Clinical characteristics and outcomes of gastrointestinal stromal tumor patients receiving surgery with or without TKI therapy: a retrospective real-world study. World J Surg Oncol 2023;21:21. [Crossref] [PubMed]
- Park N, Lim DR, Kuk JC, et al. Comparison of clinical characteristics and outcomes after surgery of gastric and small bowel GIST in single center experiences. Asian J Surg 2023;S1015-9584(23)00001-5.
- Wan W, Xiong Z, Zeng X, et al. The prognostic value of gastrointestinal bleeding in gastrointestinal stromal tumor: A propensity score matching analysis. Cancer Med 2019;8:4149-58. [Crossref] [PubMed]
- Pih GY, Jeon SJ, Ahn JY, et al. Clinical outcomes of upper gastrointestinal bleeding in patients with gastric gastrointestinal stromal tumor. Surg Endosc 2020;34:696-706. [Crossref] [PubMed]
- Cheung NK, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA 2012;307:1062-71. [Crossref] [PubMed]
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://wwwohrica/programs/clinical_epidemiology/oxfordasp
- Yin Z, Gao J, Liu W, et al. Clinicopathological and Prognostic Analysis of Primary Gastrointestinal Stromal Tumor Presenting with Gastrointestinal Bleeding: a 10-Year Retrospective Study. J Gastrointest Surg 2017;21:792-800. [Crossref] [PubMed]
- Xiao CC, Zhang S, Wang MH, et al. Clinicopathological features and prognostic factors of rectal gastrointestinal stromal tumors. J Gastrointest Surg 2013;17:793-8. [Crossref] [PubMed]
- Liu Q, Li Y, Dong M, et al. Gastrointestinal Bleeding Is an Independent Risk Factor for Poor Prognosis in GIST Patients. Biomed Res Int 2017;2017:7152406. [Crossref] [PubMed]
- Lv A, Li Z, Tian X, et al. SKP2 high expression, KIT exon 11 deletions, and gastrointestinal bleeding as predictors of poor prognosis in primary gastrointestinal stromal tumors. PLoS One 2013;8:e62951. [Crossref] [PubMed]
- Wang H, Chen P, Liu XX, et al. Prognostic impact of gastrointestinal bleeding and expression of PTEN and Ki-67 on primary gastrointestinal stromal tumors. World J Surg Oncol 2014;12:89. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am 2013;42:399-415. [Crossref] [PubMed]
- Trupiano JK, Stewart RE, Misick C, et al. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol 2002;26:705-14. [Crossref] [PubMed]
- Min YW, Park HN, Min BH, et al. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg 2015;19:631-8. [Crossref] [PubMed]
- Kang HC, Menias CO, Gaballah AH, et al. Beyond the GIST: mesenchymal tumors of the stomach. Radiographics 2013;33:1673-90. [Crossref] [PubMed]
- Nishida T, Hølmebakk T, Raut CP, et al. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann Surg Oncol 2019;26:1669-75. [Crossref] [PubMed]
- Yang ML, Wang JC, Zou WB, et al. Clinicopathological characteristics and prognostic factors of gastrointestinal stromal tumors in Chinese patients. Oncol Lett 2018;16:4905-14. [Crossref] [PubMed]


