
Evaluation of the serotonin pathway as a biomarker in cholangiocarcinoma
Highlight box
Key findings
• Serotonin (5HT) expression in cholangiocarcinoma (CCA) was absent in almost all tumor tissue specimens examined in our study while tryptophane hydroxylase-1 (TPH-1) expression was not predictive of response to cisplatin plus gemcitabine (CisGem).
What is known and what is new?
• 5HT is implicated in CCA cell proliferation and tumor growth.
• In our study, 5HT expression was absent in most tumors evaluated precluding its further evaluation as biomarker for CisGem treatment. There was a trend for improved survival in patients with high TPH-1 tumor expression and lower serum 5HT concentration.
What is the implication, and what should change now?
• The antitumor activity of 5HT production targeting with telotristat ethyl in combination with CisGem in patients with advanced CCA is active area of clinical investigation. Future translational research should include a comprehensive analysis of the 5HT pathway using techniques beyond immunohistochemistry.
Introduction
Cholangiocarcinomas (CCAs) are rare and aggressive malignant tumors of the biliary tract representing approximately 3% of all gastrointestinal cancers. CCA is commonly diagnosed in advanced stage and has a poor outcome (1). Systemic chemotherapy with cisplatin and gemcitabine (CisGem) has been the standard of care for many years for advanced CCA but still prognosis remains poor (2). Recently, the addition of immune checkpoint inhibitors to CisGem was proven to significantly improve overall survival (OS) in patients with CCA but the absolute clinical benefit was modest (3,4). In addition, there is no reliable predictive biomarker to guide patient selection. There is an unmet need for novel, biomarker-driven therapeutic strategies in CCA.
The neurotransmitter serotonin (5HT) has pleotropic effects on tumors and the immune system (5,6). Cholangiocytes can express 5HT receptors, produce and secrete 5HT that acts with autocrine and paracrine mechanisms to modulate the biliary tree remodeling and fibrosis processes (7-9). 5HT is increased in CCA, most likely a combined result of increased production from tryptophane and decreased degradation as expression of tryptophane hydroxylase (TPH) and monoaminoxidase (MAO) is increased and decreased in CCA cells vs. non-malignant cells, respectively (10), the latter potentially through an immune mechanism involving IL-6 (11). In preclinical CCA models, 5HT increased CCA cell proliferation in vitro while specific inhibition of 5HT receptors and TPH inhibition prevented and decreased proliferation of CCA respectively (10). Similarly, inhibition of 5HT synthesis in vivo decreased CCA growth (10). In benign cholestatic disease models, 5HT receptor and TPH inhibition, can decrease fibrosis and inflammation, both hallmarks of CCA (12). The antitumor effect of TPH inhibition with telotristat ethyl in combination with CisGem in patients with advanced, treatment naïve CCA is actively investigated in the ongoing TELE-ABC trial (NCT03790111, principal investigators: Renuka V. Iyer/Richard Kim). The study has completed accrual and the results are forthcoming.
Despite the preclinical evidence of detrimental effect of 5HT in CCA tumor growth, to our knowledge, the value of the expression of individual constituents of the 5HT pathway as prognostic and predictive biomarkers in CCA patients treated with current state of the art systemic therapy is unknown. We hypothesized that increased 5HT expression in CCA is associated with worse overall treatment outcomes. To test our hypothesis, we performed a single-institution retrospective analysis of 5HT and TPH expression in tumor samples and 5HT concentration in serum as predictive and prognostic biomarkers in CCA patients treated with CisGem in the first-line setting. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-115/rc).
Methods
Inclusion and exclusion criteria
In this retrospective study, we included patients ≥18 years, with locally advanced unresectable, recurrent, or metastatic CCA (including intra-, extrahepatic, gallbladder, and ampulla of Vater) who were treated with standard of care CisGem between 2006 and 2018 at Roswell Park Comprehensive Cancer Center. Patients were included only if there was available archival tumor tissue for immunohistochemistry (IHC) obtained before initiation of CisGem (either diagnostic biopsy or surgical resection material). Patients were characterized as “responders” vs. “non-responders” using RECIST 1.1 (13). Patients who had no data on response or OS were excluded as well as patients who were treated with investigational anticancer agents. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This was a retrospective study performed under an IRB-improved protocol including patients who had provided universal consent for inclusion of their tumor specimens in research studies. The study protocol was approved by Roswell Park Comprehensive Cancer Center’s Institutional Review Board (Roswell Park BDR-119819).
Study objectives and endpoints
The primary objective was to evaluate expression of 5HT and TPH in archival tumor specimens by IHC. Secondary objectives were to evaluate tissue 5HT and TPH expression and serum 5HT as prognostic and predictive biomarkers. The endpoints examined were tissue 5HT and TPH H-scores, OS, and objective response rate (ORR).
Tumor tissue analysis
Formalin-fixed paraffin (FFPE) tumor specimens from patients fulfilling the study inclusion and exclusion criteria were retrieved from the Pathology Network Shared Resource (PNSR), Roswell Park’s tumor biorepository. FFPE sections were cut at 4 µm, placed on charged slides, and dried at 60 ℃ for 1 hour. Slides were cooled to room temperature and added to the Leica Bond Rx, where they were deparaffinized with Bond Dewax Solution (Leica AR9222) and rinsed in water. Bond Epitope Retrieval 1 (Leica AR9961) was used for target retrieval for 30 minutes. Slides were blocked using peroxide block from Bond Polymer Refine Detection kit (Leica DS9800) for 5 minutes. Slides were incubated with 5HT (Leica PA0736) for 20 minutes or TPH (TPH-1; Atlas HPA022483) at 1/50 for 20 minutes. The following reagents are from the Bond Polymer Refine Detection kit (Leica DS9800). Post Primary was applied for 8 minutes followed by Polymer for 8 minutes. Diaminobenzidine (DAB) was applied for 10 minutes for visualization. Slides were counterstained with Hematoxylin for 8 minutes then placed into water. After removing slides from the Leica Bond Rx, they were dehydrated, cleared, and coverslipped. Whole section slides were digitally scanned using Aperio Scanscope (Aperio Technologies, Inc., Vista, CA, USA) with 20× bright-field microscopy. These images were accessible using Spectrum (Aperio Technologies, Inc.). Subsequently, Aperio ImageScope version 12.4.3.7009 (Aperio Technologies, Inc.) was used to view images for image analysis. An annotation layer was created for each whole section slide by selecting multiple regions of interest in the tumor tissue. Regions were identified and annotated to appropriately represent the heterogeneity of staining of each whole section and to reduce irrelevant regions from image analysis calculations. The Aperio image analysis platform was used to develop quantitative image analysis algorithm macros for the quantification of IHC. A cytoplasmic algorithm was tailored to fine tune the cell feature detection using cellular, nuclear, and stain parameters, creating a specific algorithm’s macros for 5HT and TPH based on the cell compartment location of the target protein. The cytoplasmic algorithm analyzed DAB staining intensity and the percentage of cells containing stain within the cytoplasm compartment. The algorithm results provided total number of cells, percentage per scoring intensity class, average positive intensity and H-score. The H-score is a weighted index score derived from the average intensity of the staining of the cytoplasm according to the threshold intervals set in the algorithm macro. This score equals = 1*(%1+) + 2*(%2+) + 3*(%3+) with the score between 0 and 300, where 300 represents 100% of cells being 3+. An experienced gastrointestinal surgical pathologist (LeVea CM) reviewed hematoxylin and eosin (H&E) sections for presence of neuroendocrine features and tumor-infiltrating lymphocytes (TILs). The study pathologists (LeVea CM and Bshara W) were unaware of the individual and aggregate patient outcomes.
Serum 5HT analysis
Serum samples from patients fulfilling the study inclusion and exclusion criteria were retrieved from the Roswell Park’s Data Bank and Biorepository Shared Resource (DBBR). 5HT was measured in available serum samples in a CLIA-certified laboratory using a proprietary clinical assay (Mayo Clinic Laboratories, Rochester, MN, USA). The lab personnel were unaware of the individual and aggregate patient outcomes.
Statistical analysis
Patient demographic and clinical characteristics were summarized in the overall sample using the appropriate descriptive statistics. The Spearman correlation coefficient (ρ) was used to evaluate the association between 5HT and TPH-1 expression. The association between TPH-1 expression and patient characteristics (i.e., stage, primary tumor location, and sex) was evaluated using the Mann-Whitney U exact test. The 5HT tissue scores were summarized by demographic and clinical characteristics. The 5HT serum levels were summarized by demographic and clinical characteristics. The mean, median, and standard deviation were reported with comparisons made using the Mann-Whitney U exact tests. Tissue category was then summarized by demographic and clinical characteristics. The frequencies and relative frequencies were reported and compared using the Fisher’s exact tests.
Patient characteristics and biomarker expression were also summarized by response status and compared using the Mann-Whitney U or Fisher’s exact tests, as appropriate. The biomarker expression was categorized (dichotomized at the median and terciles were considered) and associations with OS was evaluated using standard Kaplan-Meier methods and the log-rank test. Estimates of the median survival and corresponding 95% confidence intervals (CIs) were obtained.
All analyses were conducted in SAS v9.4 (Cary, NC, USA) at a significance level of 0.05.
Results
Twenty-three patients (52.2% male) fulfilled the inclusion criteria and were included in the analysis (Table 1). The median age was 59 years (range, 38–78 years). The majority were Caucasians (87%) and had intrahepatic CCA (56.5%). Almost half of patients had stage IV disease. Only 4 patients had a response to CisGem for an ORR or 17.4%. There were no statistically significant differences in baseline characteristics in responders vs. non-responders (Table 1). The median OS for the whole cohort was 17.6 months (range, 11.1–41.5 months).
Table 1
| Characteristics | Overall (n=23) | Non-responder (n=19) | Responder (n=4) | P value |
|---|---|---|---|---|
| Age (years) | 59 (38 to 78) | 57 (38 to 78) | 69 (43 to 71) | 0.68 |
| Gender | 0.32 | |||
| Female | 11 (47.8) | 8 (42.1) | 3 (75.0) | |
| Male | 12 (52.2) | 11 (57.9) | 1 (25.0) | |
| Race | >0.99 | |||
| American Indian, Aleutian, or Eskimo | 1 (4.3) | 1 (5.3) | 0 | |
| African American | 2 (8.7) | 2 (10.5) | 0 | |
| Caucasian | 20 (87.0) | 16 (84.2) | 4 (100.0) | |
| Disease stage at diagnosis | 0.48 | |||
| 1 | 3 (13.0) | 2 (10.5) | 1 (25.0) | |
| 2 | 5 (21.7) | 5 (26.3) | 0 | |
| 3 | 2 (8.7) | 2 (10.5) | 0 | |
| 4 | 11 (47.8) | 10 (52.6) | 1 (25.0) | |
| Not specified | 2 (8.7) | 0 | 2 (50.0) | |
| T stage at diagnosis | 0.77 | |||
| 1 | 4 (17.4) | 3 (15.8) | 1 (25.0) | |
| 2 | 7 (30.4) | 6 (31.6) | 1 (25.0) | |
| 3 | 4 (17.4) | 4 (21.1) | 0 | |
| 4 | 1 (4.5) | 1 (5.2) | 0 | |
| Not specified | 7 (30.4) | 5 (26.3) | 2 (50.0) | |
| N stage at diagnosis | >0.99 | |||
| 0 | 17 (73.9) | 14 (73.7) | 3 (75.0) | |
| 1 or higher | 4 (17.4) | 3 (15.8) | 1 (25.0) | |
| Not specified | 2 (8.7) | 2 (10.5) | 0 | |
| M stage at diagnosis | >0.99 | |||
| 0 | 11 (47.8) | 9 (47.4) | 2 (50.0) | |
| 1 | 11 (47.8) | 10 (52.6) | 1 (25.0) | |
| Not specified | 1 (4.3) | 0 | 1 (25.0) | |
| Primary tumor location | 0.58 | |||
| Ampulla of Vater | 2 (8.7) | 1 (5.3) | 1 (25.0) | |
| CCA, unspecified | 2 (8.7) | 2 (10.5) | 0 | |
| Extrahepatic bile duct | 2 (8.7) | 2 (10.5) | 0 | |
| Gallbladder | 4 (17.4) | 4 (21.1) | 0 | |
| Intrahepatic bile duct | 13 (56.5) | 10 (52.6) | 3 (75.0) | |
| Grade | 0.63 | |||
| II | 8 (34.8) | 7 (36.8) | 1 (25.0) | |
| III | 6 (26.1) | 4 (21.1) | 2 (50.0) | |
| Not determined | 9 (39.1) | 8 (42.1) | 1 (25.0) | |
| Histology | 0.67 | |||
| Adenocarcinoma | 8 (34.8) | 6 (31.6) | 2 (50.0) | |
| CCA | 14 (60.9) | 12 (63.2) | 2 (50.0) | |
| Mucinous carcinoma | 1 (4.3) | 1 (5.3) | 0 | |
| Alcohol use | 0.18 | |||
| Current | 12 (52.2) | 11 (57.9) | 1 (25.0) | |
| Former | 1 (4.3) | 0 | 1 (25.0) | |
| Never | 8 (34.8) | 7 (36.8) | 1 (25.0) | |
| Not reported | 2 (8.7) | 1 (5.2) | 1 (25.0) | |
| Tobacco use | 0.80 | |||
| Current | 6 (26.1) | 5 (26.3) | 1 (25.0) | |
| Former | 8 (34.8) | 6 (31.6) | 2 (50.0) | |
| Never | 9 (39.1) | 8 (42.1) | 1 (25.0) |
Data are shown as median (range) or n (%). T, tumor; N, node; M, metastasis; CCA, cholangiocarcinoma.
The median 5HT and TPH-1 H-scores were 0 (range, 0–4.93) and 170.71 (range, 100.78–273.88). There was no statistically significant correlation between 5HT and TPH-1 expression (ρ=0.16, P=0.46; Tables S1,S2). Representative sections with various expression levels of TPH-1 are presented in Figure 1. The association between 5HT and TPH-1 H-scores is presented in Figure 2 and in Tables S1,S2. Then we evaluated intensity IHC staining scores for 5HT and TPH-1. Twenty-seven and 73% of tumor specimens had 0 and +1 5HT expression by IHC respectively, while for TPH-1 the intensity was +2 in 55% of samples. Thirty-six and 9% had +1 and +3 TPH-1 expression respectively. The association between 5HT and TPH-1 staining intensity is presented in Tables S1,S2.
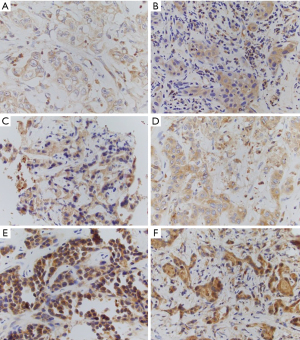

Tissue 5HT expression was low in the examined specimens and there was no association with TPH-1 expression. As the staining characteristics of TPH-1 appeared superior to 5HT, we decided to evaluate only TPH-1 as prognostic and predictive biomarker. First, we sought to determine whether TPH-1 expression differs depending on disease stage, location, and gender. There was no difference in H-score in patients with metastatic disease on presentation vs. not, intrahepatic CCA vs. other location or gender (Table 2, Table S1). Then, we evaluated the prognostic role of TPH-1. To this end, we evaluated OS by TPH-1 expression (H-score and staining intensity). There was an unexpected trend for improved OS in patients with high TPH-1 expression in tumors (Table 3). Finally, we assessed the predictive value of TPH-1 as biomarker of response or resistance to CisGem. There was no difference in TPH-1 expression in tumor tissue in responders vs. non-responders (Table 4, Table S1).
Table 2
| Characteristics | TPH H-score, mean (SD) | P value |
|---|---|---|
| Stage | 0.60 | |
| 1–3 | 186.1 (53.8) | |
| 4 | 172.1 (49.4) | |
| Primary tumor location | 0.84 | |
| Intrahepatic bile duct | 176.6 (51.9) | |
| Other | 174.9 (47.9) | |
| Sex | 0.50 | |
| Male | 170.7 (53.8) | |
| Female | 182.2 (44.9) |
TPH, tryptophane hydroxylase; SD, standard deviation.
Table 3
| OS in months, median (95% CI) | P value | |
|---|---|---|
| TPH H-score | 0.59 | |
| < Median | 17.6 (11.0, 36.4) | |
| ≥ Median | 41.5 (4.2, 106.1) | |
| Lower third | 17.0 (9.4, 29.1) | 0.77 |
| Middle third | 27.0 (0.2, 106.1) | |
| Upper third | 41.5 (4.2, 51.7) | |
| TPH intensity | 0.97 | |
| 1 | 19.8 (9.4, NR) | |
| 2/3 | 27.0 (0.2, 17.6) |
TPH, tryptophane hydroxylase; OS, overall survival; CI, confidence interval; NR, not reported.
Table 4
| Non-responder | Responder | P value | |
|---|---|---|---|
| TPH H-score | 0.80 | ||
| Mean (SD) | 176.08 (52.36) | 175.26 (37.18) | |
| Median (range) | 167.14 (100.78–273.88) | 174.89 (130.15–221.11) | |
| TPH intensity, n (%) | 0.75 | ||
| 1 | 7 (38.9) | 1 (25.0) | |
| 2 | 9 (50.0) | 3 (75.0) | |
| 3 | 2 (11.1) | 0 |
TPH, tryptophane hydroxylase; SD, standard deviation.
The finding the higher TPH-1 expression may be associated with improved outcomes contrasts what is known about 5HT role in CCA pathogenesis. The available tumor specimens were small not allowing further evaluation of 5HT pathway constituents such as MAO. In a previous study (10), increased expression of neuroendocrine markers (chromogranin A and neuron specific enolase) was present in a significant percentage of CCA suggesting a “neuroendocrine” CCA subtype. As such, we reviewed available tissue specimens for morphologic neuroendocrine features and association with TPH-1 expression. There were no neuroendocrine features in the H&E sections examined.
Since 5HT has pleotropic effects on the immune system (6), we also evaluated the H&E sections for TILs in order to see if there is an association between 5HT and TPH-1 expressions with TILs. The percentage of TILs was 0 in 17 specimens (H-scores: TPH-1 median 200; range, 100–300), 5HT median 0; range, 0–25), <1% in 2 specimens (H-scores: TPH-1 not evaluable in one and 110 in the second, 5HT 0 in both specimens), 1% in 3 specimens (H-scores: TPH-1 median 300, range, 200–300, 5HT 0 in all specimens), and 5% in one specimen (H-scores: TPH-1 200, 5HT 0). Since the number of specimens with at least 1% TILs were very small, we could not make any meaningful comparisons in between groups.
Finally, we measured 5HT in available serum samples. Only 12 patients had available serum samples for analysis. 5HT was within normal limits in all 12 samples. The differences in serum 5HT concentrations between intrahepatic CCA vs. other locations, gender, stage, and responders vs. non responders were not statistically significant, most likely secondary to very small sample size (Table S3). Additionally, there was no difference in TPH-1 or 5HT tumor tissue staining in patients with high vs. low 5HT serum concentrations (dichotomized at the median, data not shown). There was a trend towards higher concentration in patients with intrahepatic CCC, women and stage IV disease. Then, we evaluated OS in patients with high vs. low 5HT serum concentrations (dichotomized at the median). The difference in OS was not statistically significant (low: median 36.4 months, 95% CI: 9.4 to not reached, vs. high: median 23.0 months, 95% CI: 11.7–106.1, P=0.63, Figure S1). Similarly, there was no association between serum 5HT concentration and OS when 5HT was treated as continuous variable (P=0.26, HR =1.01).
Discussion
This is to our knowledge the first attempt to assess the value of the expression of constituents of the 5HT pathway as prognostic and predictive biomarkers in patients with CCA treated with CisGem. The expression of 5HT was very faint in the tumor tissues examined despite high TPH-1 expression precluding any further meaningful 5HT biomarker analyses. In addition, there was no association between 5HT and TPH-1 expression. Similarly, the concentration of 5HT was within normal limits in all analyzed serum specimens. Intriguingly, there was a trend for higher serum 5HT concentrations in patients with intrahepatic CCA, stage IV disease; there was a trend towards worse OS in patients with higher 5HT concentrations.
Beyond the limitations of evaluating small biopsy samples with small numbers of cancer cells and no adjacent non-tumor tissue as control, our tissue assay may have not been able to appropriately detect the 5HT released from tumor cells in the tumor microenvironment (TME). Furthermore, the production of 5HT may not be continuous. Physiologically, enterochromaffin cells (EC) produce 5HT in response to mechanical stress with a mechanism involving the adenosine pathway, a response that is amplified in neuroendocrine neoplasia (14). Similarly, hypoxia can stimulate 5HT production (15). Additionally, the half-life of 5HT tends to be rather short (16). Taken together, temporospatial heterogeneity and use of small biopsy samples in patients with advanced disease can limit the usefulness of 5HT as prognostic and predictive biomarker.
The expression of TPH is increased in CCA compared to normal liver (10). In our dataset, we did not identify any association between TPH-1 and primary tumor location or disease stage. Similarly, there was no indication that TPH-1 expression can be a predictive biomarker of response to CisGem. Unexpectedly, there was a trend for improved OS in patients with high TPH-1 expression. This finding should be carefully interpreted. First, increased TPH is a surrogate marker for increased 5HT production. As discussed previously, 5HT expression was neither increased in the examined tumor specimens nor there was association between TPH-1 and 5HT expressions. The overall 5HT secretion is affected by the relative balance between TPH and MAO activity. While MAO expression is decreased in CCA in contrast to normal liver (10), the limited amount of tumor tissue in the specimens evaluated in this study did not allow evaluation of MAO expression. Further, tumor TPH expression does not consider the tumor-promoting effect of 5HT released in the TME from activated platelets. In support, there was a trend for worse OS in patients with higher serum 5HT concentrations. Second, regardless of the amount of 5HT, final effects on tumor cells and TME are dependent on specific 5HT receptor expression and activity. 5HT receptor expression has been observed in CCA cells lines but the relative expression compared to normal liver cells varies in between different receptor subtypes (10). Inhibition of upregulated 5HT receptors can prevent tumor growth (10). 5HT has multiple effects on the immune system that are specific receptor dependent, some of which can be antitumoral (6). Additionally, metabolism of tryptophane via the indoleamine 2,3-dioxygenase-1 (IDO-1) and conversion into kynurenine contributes to immunosuppression and poor outcomes in CCA (17,18). Unfortunately, as with MAO, tissue was limited for IDO-1/kynurenine pathway interrogation. We postulated that improved prognosis in CCA expressing high levels of TPH and low 5HT may be associated with improved immune surveillance as manifested by high TILs but this could not be verified in the tumor specimens evaluated.
As a results of limited tumor tissue, we were not able to test our samples for neuroendocrine marker expression. Further, neuroendocrine features were not present in any of the tumor specimens examined. CCA with significant neuroendocrine component (>30% of tumor) have been reported with the neuroendocrine component mostly having characteristics of grade 2 neuroendocrine neoplasm or neuroendocrine carcinoma and localized in areas of stromal and vascular invasion and lymph node metastasis (19).
Finally, currently the treatment of CCA in patients who are pretreated with CisGem plus immune checkpoint inhibitors is guided by the molecular characteristics of the tumor, where different primary tumor locations are enriched with different targetable alterations (20). Our study is significantly limited by the small sample size, not allowing meaningful analyses within each primary tumor site and lack of molecular annotation. We must note though that the main goal of this study was to establish the baseline performance of the expression of 5HT and TPH as biomarkers in patients treated with CisGem (2) hoping that we can apply this knowledge in the future correlative tissue analysis of the TELE-ABC study (NCT03790111), were patients—similar to the landmark ABC-02 study—were enrolled regardless of primary tumor location (2).
Conclusions
In this cohort of patients with CCA treated with contemporary chemotherapy, TPH was not predictive of response to treatment but there was a trend for improved long-term outcomes in patients with high TPH expression. Tumor tissue 5HT expression was absent in most of the specimens tested. Serum 5HT levels were within normal limits in all specimens tested. There was a trend for worse survival in patients with high 5HT concentrations. Our analyses are limited by the small sample size. Finally, to better understand the role of 5HT pathway in the natural history and therapeutics of advanced CCA, the TELE-ABC trial correlative analyses will include evaluation of more constituents of the 5HT pathway using RNA sequencing rather than IHC.
Acknowledgments
Funding: The study was funded by TerSera Therapeutics, LLC. The Pathology Network and Biostatistics & Statistical Genomics Shared Resources are funded by the Roswell Park Comprehensive Cancer Center Cancer Center Support Grant (No. NCI P30CA16056).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-115/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-115/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-115/coif). RVI reports research support from TerSera Therapeutics, LLC (paid to institute, related to this work). She also reports consulting fees (paid to herself) and grants (paid to institute) from TerSera Therapeutics, LLC. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This was a retrospective study performed under an IRB-improved protocol including patients who had provided universal consent for inclusion of their tumor specimens in research studies. The study protocol was approved by Roswell Park Comprehensive Cancer Center’s Institutional Review Board (Roswell Park BDR-119819).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015;29:221-32. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;401:1853-65. [Crossref] [PubMed]
- Oh DY, He AR, Qin S, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid 2022. doi:
10.1056/EVIDoa2200015 .10.1056/EVIDoa2200015 - Masson J, Emerit MB, Hamon M, et al. Serotonergic signaling: multiple effectors and pleiotropic effects. WIREs Membr Transp Signal 2012;1:685-713. [Crossref]
- Herr N, Bode C, Duerschmied D. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med 2017;4:48. [Crossref] [PubMed]
- Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005;128:121-37. [Crossref] [PubMed]
- Omenetti A, Yang L, Gainetdinov RR, et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol 2011;300:G303-15. [Crossref] [PubMed]
- Ruddell RG, Oakley F, Hussain Z, et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol 2006;169:861-76. [Crossref] [PubMed]
- Alpini G, Invernizzi P, Gaudio E, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res 2008;68:9184-93. [Crossref] [PubMed]
- Huang L, Frampton G, Rao A, et al. Monoamine oxidase A expression is suppressed in human cholangiocarcinoma via coordinated epigenetic and IL-6-driven events. Lab Invest 2012;92:1451-60. [Crossref] [PubMed]
- Kyritsi K, Chen L, O'Brien A, et al. Modulation of the Tryptophan Hydroxylase 1/Monoamine Oxidase-A/5-Hydroxytryptamine/5-Hydroxytryptamine Receptor 2A/2B/2C Axis Regulates Biliary Proliferation and Liver Fibrosis During Cholestasis. Hepatology 2020;71:990-1008. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Chin A, Svejda B, Gustafsson BI, et al. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am J Physiol Gastrointest Liver Physiol 2012;302:G397-405. [Crossref] [PubMed]
- Dammen R, Haugen M, Svejda B, et al. The stimulatory adenosine receptor ADORA2B regulates serotonin (5-HT) synthesis and release in oxygen-depleted EC cells in inflammatory bowel disease. PLoS One 2013;8:e62607. [Crossref] [PubMed]
- Udenfriend S, Weissbach H. Turnover of 5-hydroxytryptamine (serotonin) in tissues. Proc Soc Exp Biol Med 1958;97:748-51. [Crossref] [PubMed]
- Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9:1269-74. [Crossref] [PubMed]
- Cao L, Prithviraj P, Shrestha R, et al. Prognostic Role of Immune Checkpoint Regulators in Cholangiocarcinoma: A Pilot Study. J Clin Med 2021;10:2191. [Crossref] [PubMed]
- Harada K, Sato Y, Ikeda H, et al. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch 2012;460:281-9. [Crossref] [PubMed]
- Ilyas SI, Affo S, Goyal L, et al. Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat Rev Clin Oncol 2023;20:470-86. [Crossref] [PubMed]


